Lecture 14home.gwu.edu/~chenhanning/Lecture_14.pdfthe Major Deficiency of Molecular Orbital Theory H...
Transcript of Lecture 14home.gwu.edu/~chenhanning/Lecture_14.pdfthe Major Deficiency of Molecular Orbital Theory H...
Physical Chemistry (II)
Lecture 14
CHEM 3172-80
Lecturer: Hanning Chen, Ph.D.03/20/2017
Semi-empirical Hückel Theory
the Major Deficiency of Molecular Orbital Theory
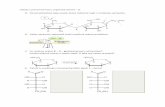
H2O molecule:
O
H2H1
atomic orbitalscO
cH1 cH2
ϕ = cO fO + cH1 fH1 + cH2 fH2molecular orbital:
fO , fH1 , fH2
HΨ = EΨ cO ,cH1 ,cH2{ }
Construction of Molecular Orbitals:
ϕn = cnij fijj=1
N
∑i=1
M
∑ atomic orbitals
A molecular orbital is a linear combination of atomic orbitals LCAO
M : the number of atomsN : the number of orbitals in each atom
hundreds or even thousands of primitive functions
construction and diagonalization of a million by million matrix
Complexity of Some Popular MO Thoeries
Correlation Theory Complexity
Configuration Interaction Single (CIS) N5
Configuration Interaction Single-Double (CISD) N6
Coupled Cluster Single-Double (CCSD) N7
Coupled Cluster Single-Double-Triple-Quadruple (CCSDTQ) N9
Second-order Moller-Plesset Perturbation (MP2) N5
Fourth-order Moller-Plesset Perturbation (MP4) N6
Hartree-Fock (HF) N4
Is there any way to avoid the expensive computational cost without losing the most important chemical and physical information ?
N : number of atoms
BenzeneBenzene: C6H6
C
C
C
C
C
CH
H
H
H
H
H
C : 1s2 2s2 2p2 H : 1s1
C
H
C C
3 σ bonds1 unpaired electron
6 π electrons
in total6 × 4 + 6 ×1= 30 valence electrons
24 σ electrons
σ πσ electrons: inner sphere
π electrons: outer sphere
chemically inert
chemically active
Is it possible to investigate the
chemically active electrons only?
12 σ bonds3 π bonds
Electron ApproximationAn atom is decomposed into a core part and a valence part:
π
Core: nucleus + electronsσValence: electronsπ
valence electron Hamiltonian:
Hπ = H jcore i( )
j=1
ncore
∑ + 1rijj=1
j<i
∑i=1
nπ
∑i=1
nπ
∑ valence-valence electronic repulsion
valence-core Hamiltonian:H j
core(i) : interaction between the i th valence electron and the j th core
ijelectron-electron repulsion ( - )π σelectron-nucleus attraction ( - N)π
Hückel Molecular Oribital Method
Hπ = H jcore i( )
j=1
ncore
∑ + 1rijj=1
j<i
∑i=1
nπ
∑i=1
nπ
∑valence electron Hamiltonian:
= 0The first assumption:
no valence-valence
repulsion !
Hπ = H jcore i( )
j=1
ncore
∑i=1
nπ
∑The second assumption:
Ψ = ϕii=1
nπ
∏ =ϕ1ϕ2...ϕnπ
Ψ : many-electron wavefunctionϕi : molecular orbitals
The anti-symmetric requirement of the many-electron wavefunction is NO LONGER satisfied !
no electron exchange interaction no electron correlation
P r1,r2( ) = P r1( )P r2( )first electron at r1second electron at r2
simultaneously product of individual probabilities
only valence-electron interactions
Linear Combination of Atomic Orbitals
H jcore i( )
j=1
ncore
∑ ϕi = Eiϕi
Solution for Hückel Hamiltonian:
C
C
C
C
C
CH
H
H
H
H
H
ϕi = cij f jj=1
nπ
∑ f j : atomic orbitalsf1
f2
f3f4
f5
f61
2
345
6
Benzene: C6H6
ϕi = ci1 f1 + ci2 f2 + ci3 f3+ci4 f4 + ci5 f5 + ci6 f6
following the procedure in Hartree-Fock theory:
HC = ESCH : Hamiltonian matrixS : Overlap matrixC : Coefficient vectorE : molecular orbital energy
the molecular orbitals, entirely dependent on
ϕi
valence− core interaction
Matrix Representation of Hückel HamiltonianHamiltonian matrix:
Hij = drfi*(r)Hπ
core∫ f j r( ) =α for i = j
β for bonded atom i and j0 for unbonded atom i and j
⎧
⎨⎪
⎩⎪
energy of non-interacting orbitalscoupling between bonded atomsno coupling between non-bonded
atomsThe third assumption:
The heterogeneity of atom types and chemical environments are ignored !
Overlap matrix:Sij = drfi
*(r)∫ f j r( ) = 1 for i = j0 for i ≠ j
⎧⎨⎪
⎩⎪The Fourth assumption:
The atomic orbitals are orthonormal !
Simplified Schrödinger equation: HC = EC
identity matrix
core-mediated electron interactions
Butadiene as an Example
Butadiene:CH2 CH CH CH2
1 2 3 4
HC = EC
α β 0 0β α β 00 β α β0 0 β α
⎛
⎝
⎜⎜⎜⎜⎜
⎞
⎠
⎟⎟⎟⎟⎟
c1c2c3c4
⎛
⎝
⎜⎜⎜⎜⎜
⎞
⎠
⎟⎟⎟⎟⎟
= E
c1c2c3c4
⎛
⎝
⎜⎜⎜⎜⎜
⎞
⎠
⎟⎟⎟⎟⎟
Secular equation:
det
α − E β 0 0β α − E β 00 β α − E β0 0 β α − E
⎛
⎝
⎜⎜⎜⎜⎜
⎞
⎠
⎟⎟⎟⎟⎟
= 0
continuant determinant
α : energy of an isolated valence electron
β : coupling energy between chemically bonded valence electrons mediated by cores
4 π electrons
α − E β 0 0β α − E β 00 β α − E β0 0 β α − E
⎛
⎝
⎜⎜⎜⎜⎜
⎞
⎠
⎟⎟⎟⎟⎟
c1c2c3c4
⎛
⎝
⎜⎜⎜⎜⎜
⎞
⎠
⎟⎟⎟⎟⎟
= 0nontrivial
solutions
General Solution of Continuant Determinantcontinuant determinant:
a b 0 0c a b 00 c a b0 0 c a
0
0
a b 0 0c a b 00 c a b0 0 c a
= a − 2 bc cos jπn +1
⎛⎝⎜
⎞⎠⎟
⎡⎣⎢
⎤⎦⎥j=1
n
∏
det
α − E β 0 0β α − E β 00 β α − E β0 0 β α − E
⎛
⎝
⎜⎜⎜⎜⎜
⎞
⎠
⎟⎟⎟⎟⎟
= α − E( ) + 2β cos( jπ4 +1
)⎡⎣⎢
⎤⎦⎥j=1
4
∏ = 0
α − E + 2β cos jπ5
⎛⎝⎜
⎞⎠⎟ = 0, j = 1,2,3,4
E1 =α +1.618βE2 =α + 0.618βE3 =α − 0.618βE4 =α −1.618β
⎧
⎨
⎪⎪
⎩
⎪⎪
decomposition
only the diagonal terms and their adjacent elements are nonzero !
β < 0
Energy Levels of ButadieneE
nerg
y
α +1.618β
α + 0.618β
α − 0.618β
α −1.618β
E1
E2
E3
E4
ΔE1→2 = −β
ΔE3→4 = −β
ΔE2→3 = −1.236β
bonding orbitals
anti-bonding orbitals
Etotal = 2 × α +1.618β( ) + 2 × α + 0.618β( ) = 4α + 4.472β
Delocalization EnergyButadiene:
CH2 CH CH CH2
artificial wall to block electron delocalizationtruncated block of ethene
secular equation
detα − E ββ α − E
⎛
⎝⎜
⎞
⎠⎟ = 0 α − E( )2 − β 2 = 0
E1 =α + β
E2 =α − β
Eethene = 2α + 2β
delocalization energy of butadiene
ΔEdelocalization = Ebutadiene − 2Eethene = 4α + 4.472β − 2 2α + 2β( ) = 0.472βButadiene is stabilized by electron delocalization !
1 2 3 4ϕi = c1 f1 + c2 f2 ϕi = c3 f3 + c4 f4
β < 0
Solutions of Molecular Orbitals
α β 0 0β α β 00 β α β0 0 β α
⎛
⎝
⎜⎜⎜⎜⎜
⎞
⎠
⎟⎟⎟⎟⎟
c1c2c3c4
⎛
⎝
⎜⎜⎜⎜⎜
⎞
⎠
⎟⎟⎟⎟⎟
= E
c1c2c3c4
⎛
⎝
⎜⎜⎜⎜⎜
⎞
⎠
⎟⎟⎟⎟⎟
E1 =α +1.618βE2 =α + 0.618βE3 =α − 0.618βE4 =α −1.618β
⎧
⎨
⎪⎪
⎩
⎪⎪
c12 + c2
2 + c32 + c4
2 = 1normalization condition:
orthonormal atomic orbitals
ϕ1 = 0.372 f1 + 0.602 f2 + 0.602 f3 + 0.372 f4ϕ2 = 0.602 f1 + 0.372 f2 + 0.372 f3 + 0.602 f4ϕ3 = 0.602 f1 − 0.372 f2 − 0.372 f3 + 0.602 f4ϕ4 = 0.372 f1 − 0.602 f2 + 0.602 f3 − 0.372 f4
⎧
⎨
⎪⎪
⎩
⎪⎪
two central atoms
two terminal atoms
two central atoms
Visualization of Molecular Orbitals
ϕ1
ϕ2
ϕ3
ϕ4
α +1.618β
α + 0.618β
α − 0.618β
α −1.618β# of nodal planes
1
2
3
4
Higher-energy orbitals have more nodal planes !horizontal molecular axis
CyclobutadieneCyclobutadiene:
C
C
C
C
H
H
H
H
12 3
4
Hückel Hamiltonian matrix:
α β 0 ββ α β 00 β α ββ 0 β α
⎛
⎝
⎜⎜⎜⎜⎜
⎞
⎠
⎟⎟⎟⎟⎟
c1c2c3c4
⎛
⎝
⎜⎜⎜⎜⎜
⎞
⎠
⎟⎟⎟⎟⎟
= E
c1c2c3c4
⎛
⎝
⎜⎜⎜⎜⎜
⎞
⎠
⎟⎟⎟⎟⎟
det
α − E β 0 ββ α − E β 00 β α − E ββ 0 β α − E
⎛
⎝
⎜⎜⎜⎜⎜
⎞
⎠
⎟⎟⎟⎟⎟
= 0
Secular equation: Circulant determinant:
a1 a2 a3 ... anan a1 a2 ... an−1an−1 an a1 ... an−2... ... ... ... ...a2 a3 a4 ... a1
shift of array elements by one position between two
adjacent rows
Expansion of Circulant Determinant
a1 a2 a3 ... anan a1 a2 ... an−1an−1 an a1 ... an−2... ... ... ... ...a2 a3 a4 ... a1
= a1 +ω ka2 +ω k2a3 + ...+ω k
n−1an( )k=1
n
∏ω k = e
2πkni
= cos 2π kn
⎛⎝⎜
⎞⎠⎟ + isin
2π kn
⎛⎝⎜
⎞⎠⎟
k = 1,2,...,n
phase factor:
det
α − E β 0 ββ α − E β 00 β α − E ββ 0 β α − E
⎛
⎝
⎜⎜⎜⎜⎜
⎞
⎠
⎟⎟⎟⎟⎟
= α − E( ) + e2πk4iβ + 0 + e
(4−1)2πk4iβ
⎛⎝⎜
⎞⎠⎟k=1
4
∏α − E + 2β cos π k
2⎛⎝⎜
⎞⎠⎟ = 0
k = 1,2,3,4
E1 =α + 2βEnergy levels:
E2 = E3 =α E4 =α − 2β
Energy Levels of CyclobutadieneE
nerg
y
α + 2β
α
α − 2β Cyclobutadiene is very unstable !
Ecyclobutadine = 2α + 2 α + 2β( ) = 4α + 4β
Eethene = 2α + 2β
ΔEdelocalization = Ecyclo − 2Eethene = 0
Benzene
C
C
C
C
C
C
H
H
H
H
f1
f2
f3f4
f5
f61
2
345
6
Benzene: C6H6 Hückel Hamiltonian matrix:
α β 0 0 0 ββ α β 0 0 00 β α β 0 00 0 β α β 00 0 0 β α ββ 0 0 0 β α
⎛
⎝
⎜⎜⎜⎜⎜⎜⎜⎜
⎞
⎠
⎟⎟⎟⎟⎟⎟⎟⎟
c1c2c3c4c5c6
⎛
⎝
⎜⎜⎜⎜⎜⎜⎜⎜
⎞
⎠
⎟⎟⎟⎟⎟⎟⎟⎟
= E
c1c2c3c4c5c6
⎛
⎝
⎜⎜⎜⎜⎜⎜⎜⎜
⎞
⎠
⎟⎟⎟⎟⎟⎟⎟⎟
6X6 circulant determinant:
α − E( ) + e2πk6iβ + 0 + e
(5−1)2πk6iβ
⎛⎝⎜
⎞⎠⎟j=1
6
∏ = 0
E1 =α + 2βE2 = E3 =α + βE4 = E5 =α − βE6 =α − 2β
⎧
⎨
⎪⎪
⎩
⎪⎪
H
H
degeneratedegenerate
Energy Levels of Benzene
E1 =α + 2β
E2 = E3 =α + β
E4 = E5 =α − β
E6 =α − 2β
ΔEdelocalization = 2 α + 2β( ) + 4 α + β( )− 3(2α + 2β ) = 2β
Benzene is very stable !
three fragments of ethene
Hückel [4n+2] RuleFor a cyclic ring molecule with 4n+2 electrons,π
it is stabilized by electron delocalization
For a cyclic ring molecule with 4n electrons,π
aromaticity
non-aromaticityanti-aromaticityit is NOT stabilized by electron delocalization