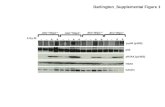
W1921 Interactions Within the p53 Protein Family Play Critical Role in Cancer Chemotherapeutic...
Transcript of W1921 Interactions Within the p53 Protein Family Play Critical Role in Cancer Chemotherapeutic...
AG
AA
bst
ract
sW1919
Ikkβ Regulates Gastric Carcinogenesis via Anti-Apoptosis and IL-1αExpressionKei Sakamoto, Shin Maeda, Hayato Nakagawa, Yoku Hayakawa, Ayako Yanai, KeijiOgura, Masao Akanuma, Masao Omata
Introduction: Nuclear factor kappa B (NF-κB) is an important transcriptional factor thatcontrols various biological processes such as inflammation, cell cycle and cell survival. NF-κB is also considered to be an important factor of carcinogenesis. However, it is still unknownwhether NF-κB activation is involved in gastric carcinogenesis. Methods: To explore theroles of IKKβ, which is the critical kinase for NF-κB activation in metastatic process, ingastric epithelium, we established conditional IKKβ knockout mouse in gastric mucosalepithelium (IKKβΔST). To induce gastric cancer in mice, we administered N-methyl-N-nitrosourea (MNU) in the weeks of 1st, 3rd, 5th, 7th, and 9th. After 8 months, we countedthe number of the tumors and measured their size. Apoptosis was analyzed by TUNELstaining. Expression of anti-apoptotic genes and levels inflammatory cytokines were meas-ured. Results: IKKβ was successfully knocked out in the gastric epithelium. There was nophenotypical and histological difference between IKKβΔST and control mice (IKKβF/F).The number of the tumors per body was significantly less in the IKKβΔST group than inthe IKKβF/F group (2.34 ± 0.48 vs. 0.80 ± 0.23; mean ± S.E.). On the other hand, the sizeof the tumors was not different between the two groups (2.75 ± 0.99 mm vs. 2.89 ± 1.12mm; mean ± S.E.). We investigated the effect of MNU administration on stomach in earlyphase (0 to 48 hours). Apoptotic cell death was significantly increased and expression ofseveral anti-apoptotic genes such as cIAPs and cFLIP were significantly reduced in IKKβΔSTcompared with the controls. The level of several inflammatory cytokines in the stomachswas measured, and IL-1α was significantly up-regulated in the IKKβΔST compared withthe controls. Other cytokines level, such as IL-1β, IL-6, or TNFα, did not change duringthe early phase. We also administered MNU by the same protocol to IL-1 receptor knockoutmice (IL-1R-/-). The number of tumors per body was less in IL-1R-/- mice than controls(1.17 ± 0.44; mean ± S.E.), and the size of the tumors was similar between the two groups(3.04 ± 1.90 mm; mean ± S.E). Conclusion: IKKβ regulates gastric carcinogenesis via anti-apoptosis and IL-1α expression. IKKβ /NF-κB signaling pathway is an attractive target forthe development of drugs for gastric cancer.
W1920
Colonic Dysplasia Is Increased By Glucagon-Like Peptide-2 in Azoxymethane-Treated Mice and Is Decreased By Antagonism of the Glucagon-Like Peptide-2ReceptorRoman Iakoubov, Lina Lauffer, Shivangi Trivedi, Karen Kai Yi Sie, Young-In J. Kim,Patricia L. Brubaker
Glucagon-like peptide-2 (GLP-2) is a nutrient-dependent intestinal hormone that promotesintestinal growth via increased proliferation and decreased apoptosis in intestinal crypt cells,as well as increasing nutrient absorption and barrier function. Therefore a long-acting GLP-2 analog (hGly2-GLP-21-33, or GLP-2) is now being tested for the treatment of short bowelsyndrome and Crohn's disease. However, since such patients are at increased risk of intestinalcancer, it is important to elucidate the potential carcinogenic effects of GLP-2. In a pilotstudy to assess the effects of chronic GLP-2 treatment, as well as of chronic inhibition ofthe GLP-2 receptor by the antagonist, GLP-23-33 (aGLP-2), on intestinal growth and prolifera-tion, 8 wk C57BL/6J mice were treated with 1 μg GLP-2, 30 ng aGLP-2, or PBS (vehicle;s.c., bid). After 4 wk, the mice were sacrificed and the intestinal tissues were collected. Smallintestinal weight/body weight was increased in the GLP-2 group (5.1±0.2% vs. 3.2±0.2%,p<0.001), and decreased in the aGLP-2 group (2.8±0.1%, p<0.05). Additionally, the colonweight/body weight was decreased in aGLP-2-treated animals (0.59±0.02% vs.0.66±0.02%,p<0.05). Furthermore, GLP-2 treatment significantly increased small intestinal villus height(706.3±16.9μm vs. 470.4±35.5μm, p<0.001) and crypt depth (82.7±1.8μm vs. 57.5±2.0μm,p<0.001), as well as the fraction of Ki67-positive, proliferating crypt cells (82.6±1.8% vs.69.8±2.5%, p<0.001). To evaluate the effects of GLP-2 and aGLP-2 on colonic dysplasia,6 wk mice were treated for 4 wk with the potent colonic carcinogen, azoxymethane (10mg/kg, i.p., q7d) , and, after 4 wk of recovery, were treated for 4 wk with GLP-2, or aGLP-2,or PBS, as in the pilot study. After an additional 12 wk, the mice were sacrificed and theintestinal tissues collected. The whole colon was then assessed for the presence of dysplasia.GLP-2-treated mice demonstrated a 1.6-fold increase in the number of colonic aberrantcrypt foci (ACF; 17.8±1.2 vs. 10.9±0.7, p<0.001). In contrast, mice treated with aGLP-2displayed a decrease in ACF counts (to 8.4±0.7; p<0.05). The findings were verified by anindependent, blinded observer. Furthermore, the ACFs in GLP-2-treated mice exhibited ahigher level of dysplasia, as demonstrated by an increase in the number of mucin-depletedfoci (1.3±0.3 vs. none, p<0.05). In summary, these studies indicate that chronic treatmentwith GLP-2 increases the appearance of cellular markers of carcinogenesis, while inhibitionof the GLP-2 receptor suppresses colonic dysplasia. These findings may have implicationsfor the long-term treatment of patients with GLP-2.
W1921
Interactions Within the p53 Protein Family Play Critical Role in CancerChemotherapeutic Treatment: New Insights Into Chemotherapeutic DrugResponse in Gastrointestinal TumorsAnna Vilgelm, Jinxiong Wei, Kay Washington, Wael M. El-Rifai, Alexander Zaika
Background: Extensive studies of the p53 tumor suppressor have demonstrated the pivotalrole this protein plays in cell cycle regulation, senescence and apoptosis. However, compre-hensive clinical analysis has found that the prognostic value of p53 mutations, thoughpromising, still does not reach the level of clinical usefulness because current methods ofassessing p53 abnormalities are not reliable and might be misleading. In fact, accumulatingdata suggest that other members of the p53 protein family, p73 and p63, strongly affectthe p53 activity but are not accounted for by mutational analysis. We explored the roles of thep53 protein family in chemotherapeutic drug response in gastrointestinal tumors. Methods:
A-754AGA Abstracts
Human colorectal tumors were analyzed by immunohistochemistry and used for isolationof primary cell cultures. Multiple cancer cell lines were treated with chemotherapeutic agentsat various concentrations and time intervals and the role of the p53 family was assessed byluciferase reporter, RT-PCR, Western blot and cell survival assays, chromatin immunoprecip-itation (ChIP), and DNA affinity immunoblotting (DAI). Stable and transient transfectionswere used for ectopic expression of p53, p63 and p73 isoforms. siRNA and dominant-negative mutants were used to inhibit the activity of p53, p73 and p63. Results: Using PCR,Western blotting and immunochistochemistry we analyzed the expression profile of theentire p53 family, and found that these proteins are expressed as a complex set of interactingisoforms. Moreover, our analyses show that interaction between multiple p53, p73 and p63isoforms play an important role in chemotherapeutic drug sensitivity. We investigated theunderlying mechanism of these interactions using DAI and ChIP assays. We found that p53,p73 and p63 interact at the level of target gene promoters. Down-regulation of both p73and p63 with dominant-negative mutants or siRNA significantly affect chemotherapeuticdrug response in cancer cells. In addition, we developed a novel approach, which allowsus to measure the functional activity of the entire p53 protein family in human tumor cells.Our analyses show that the integral activity of the p53 family is a significantly better predictorof cellular response to chemotherapeutic drug treatment than traditional analysis of p53mutations. Conclusion: We have demonstrated that chemotherapeutic drug response ismediated not only by p53 but also other members of the p53 family. Our data also suggestthat integral transcription activity of the entire p53 family can be a valuable predictor ofchemotherapeutic drug response in gastrointestinal tumors.
W1922
XAF1 Induced Mitotic Catastrophe Through Modulation of G2/M Checkpointand Interaction with Checkpoint Kinase 1Jide Wang, Wenjing Zhang, Qing Gu, Zesong Li, Liang Qiao, Hui Y. Lan, Benjamin C.Y.Wong
Background: XAF1 was first recognized as an antagonist in XIAP-suppressed caspase 3activity. It has lower expression in cancer cells than normal tissue and sensitizes TRAIL-and etoposide-triggered apoptosis. However, its role in cell growth and the underlyingmechanism have not been studied. Methods: Transfectants of gastrointestinal cancer celllines AGS, Lovo and SW1116 stably expressing XAF1 and vector control were established.Cell growth, apoptosis, morphology, mitotic status and cell cycle distribution were assessed.Mitotic catastrophe was identified by occurrence of aberrant nuclei and centrosomal ampli-fication. The expression and activity of G2/M proteins were detected by immunoblottingand kinase assay. Interaction between XAF1 and checkpoint kinase 1 (Chk1), was demon-strated by co-immunoprecipitation and the subcellular co-localization while the role of Chk1in XAF1-induced G2/M arrest was evaluated by RNA interference. Results: Over-expressionof XAF1 suppressed serum-dependent cancer cell growth, induced cell accumulation inmitosis, mitotic catastrophe andG2/M cell cycle arrest. Interestingly, XAF1was only expressedin G2/M phase after cell cycle synchronization. In addition, XAF1 interacted with andactivated Chk1, inactivated Cdc25C, and Cdc2/cyclin B complex. Suppression of Chk1abrogated XAF1-induced G2/M arrest. Furthermore, over-expression of XAF1 increased thesusceptibility of cancer cell to serum-deprivation-induced apoptosis. Conclusions: Thesefindings suggest that XAF1 promotes apoptosis secondary to mitotic catastrophe throughmodulating G2/M checkpoint, implicating a novel XIAP-independent function pathway ofXAF1. Low expression of XAF1 in cancer cell might correlate to the surveillance defect oftransformed cell in gastrointestinal tract, thus inferring XAF1 as a potential target for thetherapy of gastrointestinal cancers.
W1923
The Homeobox Transcription Factor PDX-1 Negatively Regulates the Hox-Cofactor Meis PosttranscriptionallyJohannes von Burstin, Melanie P. Wescott, Maximilian Reichert, Anil K. Rustgi
Introduction: The homeobox-protein (HOX) cofactor Myeloid ecotropic viral insertion site(MEIS) is a TALE (three-amino-acid loop extension) homeodomain transcription factor andhas several isoforms. There is growing evidence that TALE family members play a crucialrole in organ specification and cell fate determination by specifying HOX-mediated transcrip-tional gene regulation. The promoter of the pancreatic ductal specific Keratin 19 gene couldbe identified as a direct target site of MEIS1 in previous studies. Additionally, K19 genetranscription is known to be negatively regulated by the pancreatic and duodenal homeobox1 transcription factor (PDX-1). Since MEIS1 and PDX-1 are capable of interacting In Vitro(Deramaudt et al. Journal of Biological Chemistry 2006), we hypothesized that the functionalinterplay of MEIS1 and PDX-1 might be of relevance for the spatial control of K19 expressionand ductal cell fate determination in the pancreas. Methods: We used quantitative PCR forthe detection of MEIS isoforms in pancreatic ductal cells (PDCs) and the insulinoma cellline MIN6. Murine PDX-1 was subcloned into pIRES-EGFP2 and transfected into HEK 293Tcells. Serial deletions of C- and N-terminal portions of PDX-1 as well as a mutation of theprotein interaction motif (PIM), which is critical for the interaction with TALE proteins,were generated using a PCR based technique. MEIS isoforms were FLAG-tagged and clonedinto pcDNA3.1(+). Binding of MEIS to K19 promoter DNA was confirmed by EMSA. Results:We could demonstrate expression of MEIS1 and MEIS2, but not MEIS3 in PDCs by qPCR.By contrast, MEIS3 was highly expressed in PDX-1 positive/K19-negative MIN6 cells. Totest whether MEIS1 or MEIS2 levels are regulated by PDX-1, FLAG-tagged MEIS1 or MEIS2was co-transfected with PDX-1. Surprisingly, ectopic expression of PDX-1 leads to decreasedprotein levels of MEIS1 and MEIS2 without alteration of mRNA levels. To elucidate themechanism(s) which contribute to MEIS1 and MEIS2 downregulation and to clarify whichdomain of PDX-1 is involved, MEIS1 or MEIS2 was co-transfected with PDX-1 deletionmutants. We could show that deletion or mutation of the PIM in PDX-1 rescues MEISexpression. Conclusions: Posttranscriptional regulation of the HOX-Cofactor MEIS at theprotein level through the transcription factor PDX-1 may provide a novel mechanism ofdirecting cell fate during pancreatic organogenesis and in the adult pancreas. Deletion ormutation of the PIM in PDX-1 rescues MEIS protein and suggests a novel interplay betweenPDX-1 and MEIS, which is previously unappreciated.