Acetoacetic-Ester Condensation / Claisen Cond - Karstens Site
Oligopeptide-terminated poly(β-amino ester)s for highly efficient gene delivery and intracellular...
Transcript of Oligopeptide-terminated poly(β-amino ester)s for highly efficient gene delivery and intracellular...

Accepted Manuscript
Oligopeptide-terminated poly(β-amino ester)s for highly efficient gene deliveryand intracellular localization
Nathaly Segovia, Pere Dosta, Anna Cascante, Victor Ramos, Salvador Borrós
PII: S1742-7061(13)00659-4DOI: http://dx.doi.org/10.1016/j.actbio.2013.12.054Reference: ACTBIO 3058
To appear in: Acta Biomaterialia
Received Date: 24 September 2013Revised Date: 13 December 2013Accepted Date: 26 December 2013
Please cite this article as: Segovia, N., Dosta, P., Cascante, A., Ramos, V., Borrós, S., Oligopeptide-terminatedpoly(β-amino ester)s for highly efficient gene delivery and intracellular localization, Acta Biomaterialia (2014),doi: http://dx.doi.org/10.1016/j.actbio.2013.12.054
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customerswe are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, andreview of the resulting proof before it is published in its final form. Please note that during the production processerrors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.

1
Oligopeptide-terminated poly(β-amino ester)s for highly efficient gene
delivery and intracellular localization.
Nathaly Segovia, Pere Dosta, Anna Cascante, Victor Ramos*, Salvador Borrós* Grup d’Enginyeria de Materials (GEMAT), Institut Químic de Sarrià, Universitat Ramon Llull, Via Augusta 390, 08017 Barcelona *Corresponding Authors: [email protected] [email protected] Abstract
The main limitation of gene therapy towards clinics is the lack of robust, safe and efficient gene delivery vectors. Here, we describe new polycations for gene delivery based on poly(β-amino ester)s containing terminal oligopeptides. We developed oligopeptide-modified poly(β-amino ester)s – pDNA nanoparticles that achieved better cellular viability and higher transfection efficacy than other end-modified poly(β-amino ester)s and commercial transfection agents. Gene expression in highly permissive cell lines was remarkably high, however transfection efficiency in less-permissive cell lines was highly dependent on the oligopeptide composition and nanoparticle formulation. Moreover, the use of selected oligopeptides in the poly(β-amino ester)s formulation leads to preferential intracellular localization of the particles. Particle analysis of highly efficient poly(β-amino ester)s formulations revealed different particle sizes and charge features, which indicates chemical pseudotyping of the particle surface, related to the oligopeptide chemical nature. In conclusion, chemical modification at the termini of poly(β-amino ester)s with amine-rich oligopeptides is a powerful strategy to develop delivery systems for future gene therapy applications. Keywords polyplexes; gene delivery; non-viral systems; poly(β-amino ester)s; nanoparticle 1.- Introduction
The principle of gene therapy is to overcome the disorder causing the disease by direct employment of nucleic acids as a medicine. Gene therapy represents a powerful tool for the treatment of a wide range of genetic and acquired diseases. However, while this treatment approach has yielded great expectation of benefit, early clinical studies have shown disappointing failures as well as a few hints of success [1]. The major historical problem of gene therapy lies in the development of safe and efficient vectors for the delivery of therapeutic genes to their intended target cells. This goal is formidable since the human body has evolved to protect itself from environmental hazards, notably foreign nucleic acids.
Many initial efforts in gene therapy have been concentrated on the genetic engineering of viruses to develop efficient vectors for the delivery of therapeutic nucleic acids. Nowadays, viral systems are at the edge of fulfilling the short-term requirements for gene therapy and the three products approved so far (Gendicine and Oncorine in China and Glybera in EU) are based on viruses [2–4]. The basis of this success lies in their high transfection efficiency improved by years of evolution. However, the use of

2
many viral vectors is restricted by their limited packaging capacity, the cost of large-scale production and inherent safety risks [5]. Furthermore, viral vectors are often susceptible to neutralization by serum antibodies and complement, coupled with poor target-selectivity or inappropriate infectivity profile [6,7]. Synthetic vectors are likely to become an attractive choice in the future, because of their safety and easy manufacturing, however their low efficiency in delivering genetic material to target cells has limited their use as a viable treatment. A synthetic gene delivery system would be very attractive, allowing complete definition and control of the vector’s design and properties. Furthermore the manufacture of a synthetic product should be less complex and more cost effective compared to viral vectors. Nowadays, non-viral methods present some advantages over viral systems, such as a virtually infinite packaging capacity and the lack of significant immunological stimulation [8]. Although non-viral systems are still in their infancy of development, such systems are expected to play a major role in the development of new nucleic acid based therapies. As a matter of fact, an increasing number of synthetic vectors are entering clinical research in the fields of cancer [9], ophthalmology [10] and cystic fibrosis [11] management. As a result, a wide variety of non-viral vectors for the delivery of nucleic acids have been investigated, including polymers, lipids and physical methods. Typical non-viral delivery systems include lipid formulations, such as DOTAP [12], or cationic polymers, such as polyethyleneimine and poly(L-lysine) [13]. Among polymeric vectors, poly(β-amino ester)s have been described as potential non-viral polynucleotide delivery vectors capable of condensing both DNA and RNA into discrete nanometric particles [14]. Poly(β-amino ester)s have shown promise for nucleic acid delivery due to their high transfection efficiency and low toxicity [15,16]. In addition, the presence of ester bonds in their structure together with the nature of their metabolites makes them attractive materials in terms of biodegradability and biocompatibility. As a matter of fact, different types of poly(β-amino ester)s have been used successfully in a number of therapeutic applications including vaccination [17], gene therapy for cancer and ophthalmology [18–20], gene silencing [21,22] and stem cell modification [23,24]. In addition, we have recently reported the use of poly(β-amino ester)s to generate human induced pluripotent stem cells (iPS) from human fibroblasts avoiding the use of viral vectors [25]. Poly(β-amino ester)s are synthesized by Michael addition of primary amines to diacrylates. Polymers are readily synthesized without the production of any by-products and without the need of purification steps. Initial screening of large poly(β-amino ester)s libraries applying high-throughput screening approaches has prompted the identification of lead polymer structures that favor DNA complexation and transfection [26–28]. One of the best performing poly(β-amino ester) basic structures is C32 polymer, which is obtained by addition of aminopentanol to butanediol diacrylate [29,30]. In addition, post-polymerization end-modification of the resulting polymers with diamine moieties has demonstrated superior gene delivery features [15,20,29]. Interestingly, the nature of the end-modifying groups has been also described to influence transfection efficiency in a cell type dependent manner [15]. Naturally inspired vectors based on polypeptides have been earlier described and have demonstrated increased cell transfection and improved cell viability, when compared to

3
standard synthetic vectors [31,32]. However, all-peptide delivery systems are expensive to produce in large-scale quantities and may additionally elicit immune responses due to their peptidic nature. Synthetic mimics of biomacromolecules or synthetic-biologic hybrid structures may exhibit all the advantageous functional properties of their natural counterparts, but without disadvantages, such as immunogenicity, poor stability and difficulties in manufacturing [33,34]. Therefore, we describe here a new family of poly(β-amino ester) modified with oligopeptides containing basic aminoacids, i.e. arginine, lysine and histidine. We plan to demonstrate that chemical modification at the termini of poly(β-amino ester)s with amine-rich oligopeptides achieves higher transfection efficacy than other end-modified oly(β-amino ester)s pBAEs and commercial transfection agents. Following this approach, we will show that the terminal oligopeptide composition has a large influence on gene delivery performance and cell viability. In addition, our study aims to demonstrate that formulation of mixtures of oligopeptide-modified poly(β-amino ester)s is a powerful strategy to improve the gene delivery properties of these polymers.
2.- Experimental
Materials
Reagents and solvents were obtained from Sigma-Aldrich and Panreac and used as received unless otherwise stated. Plasmid pmaxGFP (3486 bp) was obtained from Amaxa. Cell lines were obtained from ATCC (Manassas, VA) and maintained at 37°C in 5% CO2 atmosphere in complete DMEM, containing 10% fetal bovine serum, 100 units/ml penicillin, 100 ug/mL streptomycin, 0.1 mM MEM Non-Essential Amino Acids (NEAA), 2 mM L-glutamine obtained from Gibco.
Synthesis of pBAEs polymers
Poly(β-aminoester)s were synthesized following a two step procedure, described in the literature. First, an acrylate-terminated polymer was synthesized by addition reaction of primary amines with diacrylates (at 1:1.2 molar ratio of amine:diacrylate). Finally, pBAEs were obtained by end-capping modification of the resulting acrylate-terminated polymer with different kind of amine- and thiol-bearing moieties. Synthesized structures were confirmed by 1H-NMR and FT-IR analysis. NMR spectra were recorded in a 400 MHz Varian (Varian NMR Instruments, Claredon Hills, IL) and methanol-d4 was used as solvent. IR spectra were obtained using a Nicolet Magna 560 (Thermo Fisher Scientific, Waltham, MA) with a KBr beamsplitter, using methanol as solvent in evaporated film. Molecular weight determination was conducted on a Hewlett-Packard 1050 Series HPLC system equipped with two GPC Ultrastyragel columns, 103 and 104 Å (5 µm mixed, 300 mm x 19 mm, Waters Millipore Corporation, Milford, MA, USA) and THF as mobile phase. The molecular weight was calculated by comparison with the retention times of polystyrene standards. Synthesis of B3 polymer
Diamine end-modified poly(β-aminoester), B3, was synthesized following a procedure described elsewhere [15,24,35]. Briefly, 5-amino-1-pentanol (3.44 g, 33 mmol) and 1,4-butanediol diacrylate (7.93 g, 40 mmol) were polymerized under magnetic stirring at

4
90°C for 24 hours. The resulting acrylate-terminated polymer C32 (1 g, 0.4 mmol) and 2-methyl-1,5-pentanediamine (0.23 g, 0.27 mL, 2 mmol) were dissolved in tetrahydrofuran and stirred overnight at room temperatures. The resulting diamine end-modified polymer B3 was isolated by precipitation in diethyl ether and dried under vacuum. IR (evaporated film): ν = 1055, 1089, 1125, 1196 (C-O), 1257, 1463, 1733 (C=O), 2079, 2191, 2253, 2861, 2936, 3398 (N-H, O-H) cm-1
1H-NMR (400 MHz, CD3OD, TMS) (ppm): δ = 4.11 (t, CH2-CH2-O), 3.72 (t), 3,55 (t, CH2-CH2-OH), 2.87 (t, -NH-CH2-CH2-C(=O)-), 2.77 (t, CH2-CH2-N-), 2.60-2.51 (br, -NH-CH2-(CH2)2-CH(CH3)-NH-), 2.46 (br, >N-CH2-(CH2)4-OH, >N-CH2-CH2-C(=O)-O), 1.87 (br), 1.73 (br), 1.60-1.41 (br, -O-CH2-CH2-CH2-CH2-O, -CH2-CH2-OH, -CH2-CH2-NH2), 1.35 (br, -N-CH2-CH2-CH2-(CH2)2-OH), 0.94 (d, CH3-CH< from diamine). Synthesis of oligopeptide-modified pBAEs polymers
In general, oligopeptide-modified pBAEs were obtained by end-modification of acrylate-terminated polymer C32 with thiol-terminated oligopeptide at 1:2.1 molar ratio in dimethyl sulfoxide. The mixture was stirred overnight at room temperature and the resulting polymer was obtained by precipitation in a mixture of diethyl ether and acetone (1:1). The following synthetic procedure to obtain tri-arginine end-modified pBAE polymer, C32-CR3, is shown as an example: A solution of C32 (0.15 g, 0.075 mmol) dissolved DMSO in (2 mL) and a solution of Cys-Arg-Arg-Arg-CONH2 · 4HCl (CR3, 0.11 g, 0.15 mmol) in DMSO (1 mL) were mixed and stirred overnight at room temperature. End-modified polymer CR3-C32-CR3 was obtained by precipitation in a mixture of diethyl ether and acetone (1:1). R3C-C32-CR3
IR (evaporated film): ν = 721, 801, 834, 951, 1029, 1133 (C-O), 1201, 1421, 1466, 1542, 1672 (C=O, from peptide amide), 1731 (C=O, from ester), 2858, 2941, 3182, 3343 (N-H, O-H) cm-1 1H-NMR (400 MHz, CD3OD, TMS) (ppm): δ = 4.41-4.33 (br, NH2-C(=O)-CH-NH-C(=O)-CH-NH-C(=O)-CH-NH-C(=O)-CH-CH2-, 4.11 (t, CH2-CH2-O), 3.55 (t, CH2-CH2-OH), 3.22 (br, NH2-C(=NH)-NH-CH2-, OH-(CH2)4-CH2-N-), 3.04 (t, CH2-CH2-N-), 2.82 (dd, -CH2-S-CH2), 2.48 (br, -N-CH2-CH2-C(=O)-O), 1.90 (m, NH2-C(=NH)-NH-(CH2)2-CH2-CH-), 1.73 (br, -O-CH2-CH2-CH2-CH2-O), 1.69 (m, NH2-C(=NH)-NH-CH2-CH2-CH2-), 1.56 (br, -CH2-CH2-CH2-CH2-OH), 1.39 (br, -N-(CH2)2-CH2-(CH2)2-OH). K3C-C32-CK3
IR (evaporated film): ν = 721, 799, 834, 1040, 1132, 1179 (C-O), 1201, 1397, 1459, 1541, 1675 (C=O, from peptide amide), 1732 (C=O, from ester), 2861, 2940, 3348 (N-H, O-H) cm-1 1H-NMR (400 MHz, CD3OD, TMS) (ppm): δ = 4.38-4.29 (br, NH2-(CH2) 4-CH-), 4.13 (t, CH2-CH2-O-),3.73 (br,NH2-CH-CH2-S-), 3.55 (t, CH2-CH2-OH), 2.94 (br, CH2-CH2-N-, NH2-CH2-(CH2)3-CH-), 2.81 (dd, -CH2-S-CH2), 2.57 (br, -N-CH2-CH2-C(=O)-O), 1.85 (m, NH2-(CH2)3-CH2-CH-), 1.74 (br, -O-CH2-CH2-CH2-CH2-O), 1.68 (m, NH2-CH2-CH2-(CH2) 2-CH-), 1.54 (br, -CH2-CH2-CH2-CH2-OH), 1.37 (br, -N-(CH2)2-CH2-(CH2)2-OH).

5
H3C-C32-CH3
IR (evaporated film): ν = 720, 799, 832, 1040, 1132, 1201, 1335, 1403, 1467, 1539, 1674 (C=O, from peptide amide), 1731 (C=O, from ester), 2865, 2941, 3336 (N-H, O-H) cm-1 1H-NMR (400 MHz, CD3OD, TMS) (ppm): δ = 8.0-7.0 (br -N(=CH)-NH-C(=CH)-) 4.61-4.36 (br, -CH2-CH-), 4.16 (t, CH2-CH2-O-), 3.55 (t, CH2-CH2-OH), 3.18 (t, CH2-CH2-N-, 3.06 (dd, -CH2-CH-), 2.88 (br, OH-(CH2)4-CH2-N-), 2.82 (dd, -CH2-S-CH2-), 2.72 (br, -N-CH2-CH2-C(=O)-O), 1.75 (br, -O-CH2-CH2-CH2-CH2-O), 1.65 (m, NH2-CH2-CH2-(CH2)2 -CH-), 1.58 (br, -CH2-CH2-CH2-CH2-OH), 1.40 (br, -N-(CH2)2-CH2-(CH2)2-OH). Nanoparticle Formation and Characterization
Polyplexes were formulated by mixing poly(β-amino esters) and pGFP plasmid at different DNA to polymer ratios (w/w). Briefly, pBAE stock solutions in DMSO (100 mg/ml) were diluted with acetate buffer (25 mM acetate buffer pH 5.0) at appropriate concentrations to obtain the desired Polymer–DNA ratios (w/w). Appropriately diluted pBAE (100 µl) was added to a solution of plasmid pGFP (100 µl at 60 µg/mL in acetate buffer 25 mM pH 5.0), mixed with vortex vigorously for few seconds and incubated at 37ºC for 30 min. The resulting nanoparticles were characterized by agarose gel electrophoresis and dynamic light scattering. To assess plasmid retardation, pBAE-DNA complexes containing 0.48 µg of pGFP at different w/w ratios were added to wells of agarose gel (0.8%, containing 1 µg/mL ethidium bromide). Samples were run at 60 V for 45 min (Apelex PS 305, France) to resolve plasmid retardation and visualized by UV illumination. Analysis of particle size distribution and zeta potential was performed in a Nanosizer ZS instrument (Malvern Instruments, UK) using pBAE-DNA polyplexes at different ratios previously diluted in phosphate buffer saline (1X).
Proton Sponge Effect – Buffering capacity
The buffer capacity of polymers was determinated by acid-base titration. Briefly, polymers were dissolved at a final concentration of 1mg/mL in an aqueous solution of sodium chloride (150 mM). The resulting polymer solution was adjusted to pH 10 with sodium hydroxide. The tritatrion curve was determined by stepwise addition of 10 µL aliquots of hydrochloric acid (0.1 M). The pH was measured after each addition with a pH meter (Crison Basic 20+, Crison Instruments) until pH 2 was reached.
GFP Transfection and Flow Cytometry
Cellular transfection was carried out using pGFP plasmid in HaCaT, hnDf, cos-7, A549 and HeLa cells. Briefly, cells were seeded on 96-well plates at 10,000 cells/well and incubated overnight to roughly 80% confluence prior to performing the transfection experiments. PBAEs–DNA complexes were prepared as earlier described using pGFP plasmid at a 50:1 polymer-to-plasmid ratio [25]. Polyplexes were diluted in serum-free DMEM medium and added to cells at a final plasmid concentration of 0.6 µg pGFP/well. Cells were incubated for 3 h at 37°C in 5% CO2 atmosphere. Subsequently, cells were washed once with PBS and complete DMEM medium was added. Cells were harvested after 48 h and analysed for GFP expression by flow cytometry (BD

6
LSRFortessa cell analyzer). GFP expression was compared against a negative control (untreated cells) and GeneJuice® (Merck KGaA, Germany) as positive control. Transfection conditions for GeneJuice® control were optimized in order to achieve maximal transfection efficiency. Different GeneJuice/pGFP ratios were evaluated and 3:1 ratio (v/w) was found to be optimal. Briefly, 300 µL serum free DMEM medium and 5.4 µL of GeneJuice were mixed thoroughly by vortexing and incubated at room temperature for 5 min. Then, 30 µL of a plasmid solution at concentration of 0.06 µg/µL in acetate buffer at 25 mM was added in to the mixture, mixed gentle by pipetting and incubated at room temperature for 15 min. Finally, 100 µL of the resulting solution were added to each well achieving a final concentration of 0.6 µg pGFP/well.
Fluorescence Microscopy of Cellular Uptake
In order to study the intracellular distribution of new end-modified pBAEs, COS-7 cells were transfected with dually-labelled nanoparticles, which were obtained from fluorescein-labelled polymers and a TAMRA-labelled oligoDNA. The new oligopeptide-modified pBAEs were mixed with 10 wt% of fluorescein-labelled polymer (B3) and the resulting mixture was used to form complexes with the TAMRA-labelled oligoDNA. The fluorescent oligonucleotide was a 20 base pair long oligonucleotide labelled with TAMRA dye at the 3’ end (5’- CAG CGT GCG CCA TCC TTC CC/36-TAMSp/-3’). Briefly, polymer:siRNA complexes at 200:1 weight ratio were prepared by mixing equal volume of oligoDNA at 0.01 µg/µL with polymers at final concentration of 2 µg/µL in AcONa buffer solution (25 mM, pH 5.5). OligoDNA was added over polymer solution and was mixed by pipetting, followed by vortexing for 5 seconds and was incubated at room temperature for 30 minutes. Polyplexes were diluted in serum-free DMEM medium and used at a final concentration of 50nM. Cells were seeded at 30,000 cells/well on microscope slides placed in 24-well plates. Cells were incubated for 3 h at 37°C in 5% CO2 atmosphere. Subsequently, cells were washed three times with PBS, stained and mounted with Vectashield mounting medium containing DAPI and were viewed in a fluorescence microscope (Zeiss Axiovert 200M). Each fluorescent stain was analysed with the corresponding filter. Blue excitation filter (420-495 nm) for green fluorescence, green excitation filter (510-560 nm) for the red fluorescence and the UV excitation filter (315-400 nm) for the blue fluorescence.
Cell Viability Assay
Cell viability assays of transfected cells were performed using the MTS assay (CellTiter 96® AQueous One Solution Cell Proliferation Assay, Promega Corporation, USA) at 48 h post-transfection as instructed by the manufacturer. Briefly, cells were transfected with pGFP plasmid as earlier described. At 48 h post-transfection, the medium was removed, cells were washed with PBS and complete medium supplemented with 20% MTS reagent (v/v) was added. Cells were incubated at 37ºC and absorbance was measured at 490 nm using a microplate reader (Elx808 Biotek Instruments Ltd, USA). Cell viability was expressed as relative percentage compared to untreated cells. 3.- Results
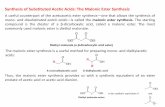
Synthesis of end-modified poly(β-amino ester)s was performed via a two-step procedure, as similarly described in previous reports (figure 1) [15,35]. First, acrylate-terminated C32 intermediate polymer was obtained by conjugate addition of 5-amino-1-

7
pentanol to 1,4-butanediol diacrylate using a slight excess of diacrylate. Polymerization was confirmed by HPLC-SEC and the resulting C32 polymer had a weight average molecular weight of approximately 2,500 g/mol (relative to polystyrene standards) and a polydispersity (Mw/Mn) of 1.81, showing a rather broad statistical distribution of polymer chain lengths. The obtained average molecular weight and polydispersity distributions are in good agreement with the polymer molecular weight achieved in previous studies at the working ratio (1:1.2 amine-to-diacrylate) [30]. C32 polymer was further modified with diamines or oligopeptide moieties, in order to obtain a new family of oligopeptide-terminated pBAEs (table 1). Diamine and oligopeptide-modified pBAEs were synthesized via addition of either one amino group of diamines or the thiol group of cysteine-terminated oligopeptides to the acrylate-terminated end-groups of C32 polymer, respectively. Both amine- and oligopeptide-terminated pBAEs were characterized in terms of molecular structure by 1H-NMR and FT-IR. In the case of B3 polymer, 1H-NMR spectrum was consistent with previously published data. The chemical structure of new oligopeptide-modified pBAEs was confirmed by the disappearance of acrylate signals and the presence of signals typically associated with aminoacid moieties. In general, both 1H-NMR and FT-IR spectra were in good agreement with the expected synthesized structures and signals from both C32 and the modifying moiety could be observed. Oligopeptide-modified pBAEs had a similar theoretical average molecular weight of approximately 3900 g/mol after modification with cysteine-terminated oligopeptides, since all polymers stemmed from the same C32 precursor.
Table 1. Synthesized oligopeptide-modified poly(β-amino ester)s and corresponding end-capping moieties.
Figure 1. Synthesis scheme of oligopeptide-terminated poly(β-amino ester)s and B3 polymer.
Proton sponge effect of oligopeptide-modified poly(β-amino ester)s
The proton sponge effect is a phenomenon that has been shown to facilitate endosomal escape and is mediated by polymers with high buffering capacity, resulting in increased transfection efficiency [36]. In general, polymers having tertiary amines in their structure show a buffering effect at the endosomal pH range between 5.0 and 7.5, which causes an increase in osmotic pressure that results in disruption of the endosome [37]. According to the proton sponge effect, the buffering capacity of the newly synthesized poly(β-amino ester)s was determined by acid titration of polymer

8
solutions (figure 2). First, the buffering effect of poly(β-amino ester) B3 was determined, showing suitable buffering capacity down to pH 5.8. The highest buffering capacity was observed with histidine-terminated poly(β-amino ester), which demonstrated high buffering in the pH range between 7.5 and 5.3. Lysine-modified poly(β-amino ester)s presented suitable buffering capacity until pH 5.9. In contrast, poly(β-amino ester)s end-capped with arginine oligopeptides only showed limited buffering capacity in the range between 7.4 and 6.4. Since all polymers stem from the same acrylate-terminated pre-polymer C32, the additional buffering capacity observed results from the amine-rich terminations. The different buffering capacity profiles of the new poly(β-amino ester)s correlated well with the different pKa’s of the amino acid side chains (∼ 12, 10 and 6 for arginine, lysine and histidine, respectively) present in the modifying oligopeptides. The highest buffering capacity observed for histidine-terminated poly(β-amino ester)s in the endosomal pH range is caused by the lower pKa of the imidazole ring of histidine, acting as a weak base. In contrast, the side chains of arginine and lysine have higher pKa values, making them less efficient at buffering at endosomal pH. These results demonstrate, as expected, that the buffering capacity of the polymers can be controlled by the amio acid composition of the modifying oligopeptides.
Figure 2. Proton sponge effect. Titration curves of poly(β-amino ester)s at 1 mg/mL. Polymer solutions were adjusted to pH 10 and subsequently titrated with HCl 0.1 M until pH 2. Control refers to titration of a solution that does not contain polymer. Complexation of oligopeptide-modified poly(β-amino ester)s with pGFP plasmid
The DNA-binding capability of the newly synthesized poly(β-amino ester)s polymers was evaluated by agarose gel electrophoresis at different polymer-to-DNA ratios (w/w) (figure 3). Complexes prepared with B3 polymer and pGFP plasmid revealed substantial free DNA at ratios below 25:1, while at ratios equal or higher than 25:1 complete DNA retention was observed. Arginine- and lysine-terminated poly(β-amino ester)s showed complete DNA retardation at 10:1 pBAE-GFP ratios. In contrast, when DNA was complexed with histidine-terminated polymers, polymer-plasmid ratios of 25:1 or higher were required to fully impede DNA mobility. In addition, complexes prepared with histidine-modified poly(β-amino ester)s revealed high fluorescence in the wells, which probably indicated inefficient DNA complexation. These results suggest that poly(β-amino ester)s containing arginine or lysine decreased significantly the pBAE/DNA ratios required for efficient complexation.
Figure 3. Gel retardation assay of poly(β-amino ester)s-DNA polyplexes. Polyplexes were formed using pmaxGFP and different poly(β-amino ester)s at indicated w/w ratios and loaded onto an agarose gel containing ethidium bromide to assess DNA mobility by electrophoresis. The complexation of DNA with poly(β-amino ester)s was also assessed using photon correlation spectroscopy, in order to characterize the size and zeta potential of the

9
resulting polymer-DNA complexes (figure 4). Complexes prepared with B3 polymer and pGFP plasmid at 50:1 ratio formed polyplexes with a particle average size of approximately 340 nm and positive zeta potential. Particles obtained from end-modified poly(β-amino ester)s with arginine-oligopeptide R3C-C32-CR3 presented a significantly smaller average particle size of approximately 210 nm, and a considerably higher positive zeta potential, when compared to B3 control. Lysine-terminated poly(β-amino ester)s formed considerably larger particles of approximately 400 nm with positive zeta potential upon complexation with DNA. In contrast, polyplexes obtained from poly(β-amino ester)s end-capped with histidine were considerably smaller than B3, however their zeta potential was markedly negative and reached -30 mV. Polyplexes prepared with mixtures of oligopeptide-modified poly(β-amino ester)s resulted in notably larger nanoparticles and lower zeta potential values, when compared to B3 control. In general, oligopeptide-containing poly(β-amino ester)s formed complexes with average hydrodynamic diameters between 200 and 400 nm and zeta potential values ranging from nearly neutral to highly positive, which is compatible with known cell entry mechanisms [38–40]. However, poly(β-amino ester)s bearing histidine-moieties, H3C-C32-CH3, formed complexes with markedly negative zeta potential values, which has been classically associated with low transfection capacity [40–43].
Figure 4. Average size and zeta potential determined by photon correlation spectroscopy. Polyplexes were prepared using pmaxGFP and different poly(β-amino ester)s at 50:1 w/w ratios and diluted in phosphate buffered saline. Results are shown as mean and standard deviation of triplicates. Statistical significance of size values was determined using B3 polymer as control group. * p<0.05, ** p<0.01
Transfection of COS-7 cells with green fluorescent protein plasmid
Transfection experiments were performed with all synthesized poly(β-amino ester)s in order to determine the influence of oligopeptide termination on transfection efficiency. Transfection was evaluated using COS-7 cells and a plasmid coding for the reporter green fluorescent protein (pmaxGFP). GeneJuice®, which is a proprietary optimized formulation, composed mainly of a non-toxic cellular protein and a small amount of polyamine, and amine-terminated B3 polymer were used as transfection controls in order to compare the transfection efficiency of newly synthesized poly(β-amino ester)s against peptide-based or all synthetic delivery vectors, respectively. GeneJuice® and B3 formulations were previously optimized in order to achieve maximal transfection efficiency (data not shown). Reporter green fluorescent protein expression was determined by flow cytometry analysis at 48h post-transfection. Data shown in figure 5 demonstrate that poly(β-amino ester)s end-modified with oligopeptides containing either arginine (R3C-C32-CR3) or lysine (K3C-C32-CK3) achieved higher transfection efficiencies, when compared to B3 poly(β-amino ester) and GeneJuice controls. Arginine- and lysine-modified pBAEs showed a 36% and 14% increase in GFP expression, respectively, when compared to B3 polymer. In contrast, poly(β-amino ester)s end-modified with histidine oligopeptides, H3C-C32-CH3, showed low levels of GFP expression, achieving less than 1% of GFP-positive cells.

10
In addition to single end-modified poly(β-amino ester)s, we were interested to determine whether mixtures of different end-modified poly(β-amino ester)s could provide an advantage in terms of transfection efficacy. For this reason, mixtures of oligopeptide-modified poly(β-amino ester)s were evaluated for their transfection efficiency. Results demonstrated that 1:1 (w/w) mixtures of arginine-, lysine- or histidine-modified poly(β-amino ester)s achieved higher transfection efficiencies than B3 control. Specially, mixtures of arginine-histidine (R/H 1:1) and lysine-histidine (K/H, 1:1) poly(β-amino ester)s showed the highest GFP expression of all tested polymer combinations, resulting in 81% and 60% increase in transfection efficiency relative to B3 control, respectively. In contrast, combination of arginine- and lysine-modified (R/K 1:1) poly(β-amino ester)s achieved only slightly higher values of GFP expression, when compared with B3 control.
Figure 5. COS-7 cells transfected with different poly(β-amino ester)s and pmaxGFP at 50:1 ratio. a) GFP expression was determined after 48 h by flow cytometry and plotted as percentage of GFP-positive cells multiplied by the mean fluorescence of the positive population. Percentage numbers above each bar represent the average percentage of GFP positive cells. Results are shown as mean and standard deviation of triplicates. Statistical significance was determined using B3 polymer as control group. * p<0.05, ** p<0.01, *** p<0.001
b) Fluorescent images of GFP expression in COS-7 cells, (i) control cells, (ii) GeneJuice, (iii) B3, (iv) R3C-C32-CR3, (v) K3C-C32-CK3, (vi) H3C-C32-CH3, (vii) R/H, (viii) K/H, (ix) R/K Cellular uptake of fluorescently labeled complexes
In order to explain the differences in GFP expression, the cellular uptake of complexes formed with oligopeptide-modified poly(β-amino ester)s was followed via fluorescent microscopy. For this purpose, poly(β-amino ester)s were labeled with a fluorescein derivative and a TAMRA-labeled non-coding oligonucleotide was used instead of the pmaxGFP plasmid. Cells were transfected with the fluorescently labeled polyplexes as described earlier with the only difference being that cells were immediately fixed after transfection and their nuclei stained with DAPI (figure 6).
Figure 6 Fluorescent images of COS-7 cells transfected with fluorescently labeled polyplexes, (i) control cells, (ii) B3, (iii) R3C-C32-CR3, (iv) K3C-C32-CK3, (v) H3C-C32-CH3, (vi) R/H, (vii) K/H, (viii) R/K. Red: DNA oligonucleotide labeled with texas red/cy3, Green: poly(β-amino ester)s labeled with fluoresceine, Blue: nuclei stained with DAPI In general, complexes obtained from oligopeptide-modified poly(β-amino ester)s showed higher cellular uptake than B3 control, except for histidine-terminated poly(β-amino ester)s. Interestingly, complexes formed from single oligopeptide-modified poly(β-amino ester)s showed different cellular distribution depending on their compositions. For instance, arginine-modified poly(β-amino ester)s showed preferential localization around the perinuclear region, while complexes formed with poly(β-amino ester)s end-modified with lysine or histidine oligopeptides were preferentially observed

11
in the cytoplasm or around the cellular membrane, respectively. Complexes prepared with mixtures of oligopeptide-modified poly(β-amino ester)s also showed different cellular localization depending on their chemical nature. Similarly as observed for arginine-modified poly(β-amino ester)s, mixtures of arginine-histidine (R/H 1:1) poly(β-amino ester)s showed high perinuclear localization, however the addition of hisitidine oligopeptides seemed to increase the number of fluorescently labeled complexes. In contrast, mixtures of lysine-histidine (K/H 1:1) poly(β-amino ester)s did not show any visually appreciable difference respect to lysine-modified poly(β-amino ester)s alone and were mainly observed in the cytoplasm. Interestingly, complexes obtained from mixtures of arginine-lysine (R/K 1:1) poly(β-amino ester)s showed both cytoplasmic and perinuclear localization. Viability of cells transfected with oligopeptide-terminated poly(β-amino ester)s
Transfection of cells using polycations is usually accompanied by some degree of cell toxicity, which may limit their use as gene delivery vectors [44]. Although the biocompatibility of cationic polymers depends on different factors, such as the density and the nature of amines or the polymer structure, in general, high molecular weight polymers having high densities of primary amines usually result in considerable cytotoxicity [44,45]. In order to evaluate the effect of oligopeptide end-modification on the biocompatibility of these materials, the cytotoxicity of oligopeptide-terminated was assessed by evaluating cell viability using the MTS assay at the endpoint of a transfection experiment. The cytotoxicity of the best performing poly(β-amino ester)s based on their transfection efficiencies assessed in the previous experiment was assayed and compared against GeneJuice and B3 polymer controls. Transfection-mediated cytotoxicity was expressed as percentage of cell viability relative to untreated cells (figure 7).
Figure 7. Cell viability levels of cells after transfection with poly(β-amino ester)s measured using the MTS assay. Viability was determined at 48 h post-transfection and plotted as percentage of viable cells relative to a control of untreated cells. Results are shown as mean and standard deviation of triplicates. Statistical significance was determined using GeneJuice as control group. * p<0.05, ** p<0.01, *** p<0.001
Transfection of COS-7 cells with GeneJuice resulted in cellular viabilities above 85%, which is in good agreement with the expected values provided by the supplier. In contrast, cells transfected with B3 poly(β-amino ester) control presented substantial cytotoxicity, resulting in a viability reduction of approximately 90%, when compared to untreated cells. Oligopeptide-modified poly(β-amino ester)s showed considerably less cell toxicity after transfection than diamine-terminated B3 polymer. In general, cells transfected with oligopeptide-modified poly(β-amino ester)s presented cell viabilities above 75%. Interestingly, the mixture of lysine- and histidine-terminated poly(β-amino ester)s (K/H) showed the lowest levels of toxicity, achieving a 98% of cell viability after transfection, even at high concentration rates and prolonged incubation times .

12
Transfection of different cell lines using oligopeptide-modified poly(β-amino ester)s
We next analyzed the transfection of GFP plasmid complexed with the top performing polymers according to the prior experiment in a panel of five different cell lines (figure 8). Among the polymers evaluated in figure 5, arginine-modified poly(β-amino ester) (R3C-C32-CR3) and three mixtures containing 1:1 combinations of arginine-, lysine- and histidine-modified poly(β-amino ester)s were chosen as the most promising polymers for transfection comparison in different cell lines. From the evaluated cell lines, COS-7 cells showed the highest permissiveness to transfection as a result of the high GFP expression observed. For A549 cells, B3 poly(β-amino ester) showed notable GFP expression, when compared to GeneJuice, indicating that poly(β-amino ester)s might be promising materials for transfection of this type of cells. However, DNA complexes prepared with arginine-modified or a mixture of arginine- and lysine-modified (R/K) poly(β-amino ester)s provided significantly lower levels of GFP expression than B3 control. Interestingly, transfection of A549 cells with mixtures of histidine-modified poly(β-amino ester) and either arginine- or lysine-modified (R/H or K/H) poly(β-amino ester)s was significantly enhanced, achieving respectively 2.4-fold and 1.9-fold increases in GFP expression, compared to B3 polymer. HeLa cell line, which is a hard to transfect cell line, showed only modest low GFP expression, when transfected using both B3 poly(β-amino ester) and GeneJuice. Similarly, complexes prepared from a mixture of arginine- and lysine-modified poly(β-amino ester) did not result in significantly greater GFP expression. However, arginine-modified poly(β-amino ester) and a mixture of lysine- and histidine-modified poly(β-amino ester) showed a notable increase in transfection efficiency, resulting in 2.9-fold and 3.6-fold increase in gene expression, when compared to B3 control. Interestingly, up to 9-fold higher levels of GFP expression were observed in this cell line, when pGFP plasmid was complexed using a mixture of arginine- and histidine-modified poly(β-amino ester)s. The fibroblast cell line (NHDF) was the less permissive cell line to transfection in view of their low transfection levels achieved for all tested polymers, when compared with the other cell lines. However, arginine-modified poly(β-amino ester) and an arginine- and lysine-modified (R/K) poly(β-amino ester) mixture were able to greatly enhance transfection, resulting in 3.7-fold and 4.1-fold increase in GFP expression respectively, relative to transfection levels achieved with B3 control poly(β-amino ester). Ultimately, HaCaT cells were evaluated for plasmid GFP transfection using the newly synthesized poly(β-amino ester)s. Interestingly, this cell line showed better transfection efficiency using Genejuice rather than B3 polymer, which may indicate that HaCaT cells are barely transfectable by the poly(β-amino ester)s. However, complexes prepared with arginine-terminated poly(β-amino ester) and a mixture of arginine- and lysine-modified poly(β-amino ester)s demonstrated high transfection efficiencies on this cell line, resulting in 14.3-fold and 11.2-fold increase in GFP expression respectively, when compared to B3 control. In contrast, polymer mixtures prepared from either arginine- or lysine-modified poly(β-amino ester)s and histidine-modified poly(β-amino ester)s showed similar levels of GFP expression to B3 polymer.

13
Figure 8. Five cell lines transfected with pmaxGFP and different poly(β-amino ester)s. GFP expression was determined after 48 h by flow cytometry and plotted as percentage of GFP-positive cells multiplied by the mean fluorescence of the positive population. Results are shown as mean and standard deviation of triplicates. Statistical significance was determined using B3 polymer as control group. * p<0.05, ** p<0.01, *** p<0.001
4.- Discussion
Conventional gene delivery agents based on cationic lipids and polymers are considerably efficient at delivering nucleic acids in vitro, however their biocompatibility and target specificity require further development in order to meet clinical expectations. Recent advances in polymer synthesis, have led to the convergence of synthetic and natural polymers resulting in new materials with improved biocompatibility and gene delivery properties [33,34,46–48]. In addition, high transfection efficacy and intrinsic biomaterial-mediated cell specificity in vitro have been described for synthetic delivery systems based on poly(β-amino ester)s [49,50]. Although these recent developments show high promise towards clinical success of nanotechnology for gene therapy, there is still urge to develop safer gene delivery systems with higher efficacy and target specificity, especially for in vivo applications. We have here developed a new family of bioinspired polymers designed for efficient delivery of nucleic acids based on oligopeptide-modification of poly(β-amino ester)s with improved gene delivery features. In our approach, we have modified acrylate-terminated poly(β-amino ester)s using thiol-ene chemistry instead of previously described amine addition, allowing the synthesis of poly(β-amino ester)s end-modified with basic oligopeptides, which would be otherwise difficult to achieve. End-capping of poly(β-amino ester) intermediate C32 with cysteine-terminated oligopeptides proceeded via thiol addition to terminal acrylate groups in a rapid and efficient manner [51]. The higher nucleophilicity of thiols made the reaction to occur more readily and required less excess of capping moieties than the standard procedure described for end-modification of poly(β-amino ester)s with diamines [52]. Conjugation of oligopeptides consisting of basic aminoacids, i.e. arginine, lysine and histidine, rendered poly(β-amino ester)s capable of efficiently condensing plasmid DNA into discrete nanometric particles with average diameters comprised between 200 and 400 nm and average zeta potentials ranging from negative to positive. Characterization of oligopeptide-modified poly(β-amino ester) polyplexes revealed that the chemical nature of the capping aminoacids had a great influence on the final physicochemical properties of the resulting nanoparticles and ultimately determined their gene delivery properties. Transfection of the highly permissive COS-7 cell line with oligopeptide-modified poly(β-amino ester)s and a plasmid encoding green fluorescent protein (GFP) revealed different gene expression efficiencies depending on the structure of poly(β-amino ester)s and the nature of the end-capping moieties. Lysine- and histidine-terminated poly(β-amino ester)s did not improve transfection efficiency, when compared to earlier described poly(β-amino ester)s. In contrast, poly(β-amino ester)s bearing arginine terminations were significantly more efficient at transfecting cells than similar polymers

14
terminated with lysine or histidine residues. The high transfection efficiencies achieved for arginine-modified poly(β-amino ester)s correlated well with the optimal physicochemical properties observed for their polyplexes, i.e. highly positively charged particles with an average hydrodynamic diameter around 100 - 200 nm [43,53]. However, increased transfection may not be only explained by the physicochemical properties of the particles, since polyplexes obtained from lysine-modified poly(β-amino ester)s showed similar features and had similar transfection levels as positive controls. Increased transfection is likely to be a consequence of increased cellular uptake, rather than increased endosome escape, since poly(β-amino ester)s end-capped with arginine presented considerably limited buffering capacity (as shown in figure 2). This is supported by the fact that when arginine-terminated poly(β-amino ester)s were formulated with histidine-terminated poly(β-amino ester)s, which possess high buffering capacity, increased gene expression levels were observed. Oligoarginine peptides comprised between 9 and 15 arginine units have been described in the literature to mediate transfection by increasing cellular uptake and nuclear trafficking [54][55]. It has been suggested that R9-R15 peptides have an optimal length capable of adopting an appropriate confirmation that induces cell entry via a poorly understood mechanism, whereas either shorter or longer poly-arginines show a partial or even complete reduction in transfection efficiency [56][57]. In addition, oligoarginine modification with hydrophobic moieties has shown to increase the efficiency of transfection significantly, probably due to the formation of supramolecular micellar structures, which promote multivalent interactions between oligoarginines and the cell surface [58]. Furthermore, surface decoration of PAMAM and poly-lysine dendrimers with arginine residues have proven to be a successful strategy to improve gene expression considerably [59,60]. Interestingly, we have observed a notable increase in reporter gene expression with just three repeats of arginine at both termini of polymer chains. This increase is likely to be caused by an increase in cellular uptake and especially due to increased preferential perinuclear localization of DNA particles as shown by fluorescence microscopy. It is likely that arginine oligomers projected from the surface of polyplexes act cooperatively through multivalent interactions with the cell surface and promote cell entry. We have observed that particles formed from mixtures of poly(β-amino ester)s showed increased transfection efficiencies, indicating that, in addition to appropriate choice of the end-capping group, which has been earlier described to determine transfection efficiency [61,62], formulation of two or more polymers may be a powerful strategy to increase gene delivery properties. In this regard, arginine- and lysine-terminated poly(β-amino ester)s achieved notable gene expression per se, however when these polymers were formulated in combination with histidine-modified poly(β-amino ester)s, the transfection capacity of the resulting mixture was significantly further increased. It is highly remarkable that, while complexes prepared from arginine- or lysine-terminated poly(β-amino ester)s alone showed standard particle features regarded as optimal for gene delivery, such as ∼100 - 200 nm in size and markedly positive zeta potential, complexes obtained from mixtures of histidine- and either lysine- or arginine-modified poly(β-amino ester)s presented average hydrodynamic diameters above 400 nm and nearly neutral or even slightly negative zeta potential, respectively. These values of size and zeta potential have been classically regarded as poor features for efficient gene delivery, since large particles may not be compatible with endocytosis and barely

15
charged particles may not interact sufficiently with the cell surface to mediate cellular uptake. This phenomenon is particularly evident in formulations made of arginine- and histidine-modified poly(β-amino ester)s, which proved very efficient at transfecting COS-7 cells, despite resulting in relatively large complexes with slightly negative zeta potential. Similarly, formulations made of lysine- and histidine-modified poly(β-amino ester)s, which on their own showed similar or even lower gene expression levels than B3 control poly(β-amino ester), achieved excellent transfection efficiency. In this particular case, it is likely that lysine-modified poly(β-amino ester) confers sufficient positive charges for DNA complexation, and histidine-modified poly(β-amino ester) introduces high buffering capacity for efficient endosomal escape. In contrast, mixtures of arginine- and lysine-modified poly(β-amino ester)s formed positively charged polyplexes with appropriate size for gene delivery, however they achieved gene expression levels that are in between of the values observed for their counterparts alone, indicating that superior transfection is not always achieved via polymer formulation. Therefore, accurate formulation of poly(β-amino ester) mixtures is required in order to synergistically combine polymer-specific features, similarly as previously described for lipid formulations for gene delivery [63,64]. It has been recently reported that for larger particles, i.e. > 400 nm, sedimentation can occur, leading to higher transfection or at least increased cellular uptake in vitro due to an increase in the local concentration of particles near the cell surface [65]. Although some of the formulations described in this work presented particles, whose properties made them susceptible for sedimentation, i.e. large particles and nearly neutral zeta potential, cellular uptake results did not show a clear correlation. In fact, mixed formulations resulting in larger particles, such as mixtures of histidine- and either arginine- or lysine-terminated poly(β-amino ester)s, showed high levels of gene expression that did not necessarily correlate with higher cellular uptake. Interestingly, fluorescence microscopy revealed that oligopeptide specific features, such as perinuclear localization for arginine oligopeptides, are maintained when formulated with other oligopeptide-modified polymer, suggesting that the properties of each oligopeptide-terminated poly(β-amino ester) are additive. This is of great interest for the design of tailored complexes having specific features, such as preferential intracellular localization or enhanced endosomal escape. Although sophisticated cationic polymers bearing multiple functionalities to increase gene delivery have been described [66–68], little research has been developed in the area of polymer formulations for more efficient gene delivery [69,70]. Our data indicates that this might be a powerful strategy to conveniently increase transfection efficiencies of synthetic vectors. Based on gene expression and cell viability levels of COS-7 cells transfected with the above described poly(β-amino ester)s, a pool of highly efficient poly(β-amino ester) candidates were selected to assess their gene delivery properties on different cell lines. Selected poly(β-amino ester) formulations showed differential gene expression levels depending on the cell line, which revealed that the chemical composition of poly(β-amino ester)s had a deep effect on transfection. While the selected poly(β-amino ester) formulations showed consistently high levels of gene expression in COS-7 cells, certain correlations between cell-specific gene expression and both the composition and surface characteristics of the resulting polyplexes could be deduced. In this regard, keratinocyte (HaCaT) and fibroblast (NHDF) cell lines, but not A549 or HeLa cell lines, showed high levels of gene expression only with polyplexes having a

16
remarkable positive charged and containing arginine-modified poly(β-amino ester)s in their composition. In contrast, lung epithelial cells (A549) were only efficiently transfected with formulations containing histidine-terminated poly(β-amino ester)s, which resulted in rather large (above 400 nm) and barely charged polyplexes. Furthermore, transfection specificity was more obvious in cervix epithelial (HeLa) cell line, as only polyplexes prepared from arginine- and histidine-modified poly(β-amino ester)s showed high gene expression levels. These results suggest that fine tunning of the chemical composition and both the size and the surface charge of polyplexes may be a powerful strategy to target gene expression to specific cell lines. Although at this time the exact mechanism, i.e. preferential cellular uptake, endosomal escape or nuclear trafficking, that benefits from poly(β-amino ester) formulation to mediate cell-specific increased gene expression is not clear, our hypothesis is that both the surface characteristics and composition play a major role on cellular uptake by specifically interacting with the cell surface. It has been recently reported that different types of human cells show wide variations in their zeta potential values [71,72]. This difference is presumably attributed to differences in the biochemical composition of the cell plasma membrane. In this regard, it is likely that depending on the glycosaminoglycan nature and structure of cells [73,74], certain polyplex features may be more suitable to interact with the cell surface and subsequently mediate internalization in a more efficient way. Therefore, we envisage that tailored polymer formulations may be regarded as a surrogate of viral pseudotyping for synthetic vectors in order to control gene expression and cell-specificity. Nonetheless, it is likely that the composition of polyplexes influences other gene delivery processes to some extent, such as both endosomal escape and nuclear trafficking. 5.- Conclusions
We have developed a new family of bioinspired polymers based on oligopeptide-modified poly(β-amino ester)s that has demonstrated efficient delivery of nucleic acids. Our approach of adding oligopeptides to the termini of poly(β-amino ester)s to improve transfection activity should provide a more cost-effective alternative to complex peptide-based vectors without the biocompatibility concerns of entirely synthetic systems. As a consequence of their rich oligopeptide functionality, these new materials and probably their degradation products behave in ways that are more biocompatible to cells, resulting in excellent cellular viability and high gene expression levels. In addition, polymer formulations described here are capable of efficiently deliver nucleic acids specifically to cells in vitro without the need of introducing ligand-mediated mechanisms. Selected poly(β-amino ester) formulations showed differential gene expression levels depending on the cell line, which revealed that the chemical composition of poly(β-amino ester)s had a deep effect on transfection. It should be highlighted, that oligopeptide specific features, such as perinuclear localization for arginine oligopeptides, are maintained when formulated with other oligopeptide-modified polymer, suggesting that the properties of each oligopeptide-terminated poly(β-amino ester) are additive. This is of great interest for the design of tailored complexes having specific features, such as preferential intracellular localization or enhanced endosomal

17
escape. Therefore, accurate formulation of poly(β-amino ester) mixtures is required in order to synergistically combine polymer-specific features. In summary, our results indicate, for the first time, that appropriate formulation of poly(β-amino ester)s can drastically affect transfection efficiencies in a cell-type specific manner. 6.- References
[1] Kay MA. State-of-the-art gene-based therapies: the road ahead. Nat Rev Genet 2011;12:316–28.
[2] Räty JK, Pikkarainen JT, Wirth T, Ylä-Herttuala S. Gene therapy: the first approved gene-based medicines, molecular mechanisms and clinical indications. Current Molecular Pharmacology 2008;1:13–23.
[3] Ylä-Herttuala S. Endgame: glybera finally recommended for approval as the first gene therapy drug in the European union. Molecular Therapy : the Journal of the American Society of Gene Therapy 2012;20:1831–2.
[4] Guo J, Xin H. Splicing Out the West? Science 2006;314 :1232–5.
[5] Kamen A, Henry O. Development and optimization of an adenovirus production process. The Journal of Gene Medicine 2004;6:S184–S192.
[6] Carlisle RC, Benjamin R, Briggs SS, Sumner-Jones S, McIntosh J, Gill D, et al. Coating of adeno-associated virus with reactive polymers can ablate virus tropism, enable retargeting and provide resistance to neutralising antisera. The Journal of Gene Medicine 2008;10:400–11.
[7] Lyons M, Onion D, Green NK, Aslan K, Rajaratnam R, Bazan-Peregrino M, et al. Adenovirus Type 5 Interactions with Human Blood Cells May Compromise Systemic Delivery. Mol Ther 2006;14:118–28.
[8] Mastrobattista E, van der Aa MAEM, Hennink WE, Crommelin DJA. Artificial viruses: a nanotechnological approach to gene delivery. Nat Rev Drug Discov 2006;5:115–21.
[9] Tabernero J, Shapiro GI, Lorusso PM, Cervantes A, Schwartz GK, Weiss GJ, et al. First-in-Man Trial of an RNA Interference Therapeutic Targeting VEGF and KSP in Cancer Patients with Liver Involvement. Cancer Discovery 2013.
[10] Nguyen QD, Schachar RA, Nduaka CI, Sperling M, Basile AS, Klamerus KJ, et al. Dose-Ranging Evaluation of Intravitreal siRNA PF-04523655 for Diabetic Macular Edema (the DEGAS Study). Investigative Ophthalmology & Visual Science 2012;53 :7666–74.
[11] Alton EWFW, Boyd AC, Cheng SH, Cunningham S, Davies JC, Gill DR, et al. A randomised, double-blind, placebo-controlled phase IIB clinical trial of repeated application of gene therapy in patients with cystic fibrosis. Thorax 2013.
[12] Simões S, Filipe A, Faneca H, Mano M, Penacho N, Düzgünes N, et al. Cationic liposomes for gene delivery. Expert Opin Drug Deliv 2005;2:237–54.
[13] Eliyahu H, Barenholz Y, Domb AJ. Polymers for DNA Delivery. Molecules 2005;10:34–64.
[14] Green JJ, Langer R, Anderson DG. A Combinatorial Polymer Library Approach Yields Insight into Nonviral Gene Delivery. Acc Chem Res 2008;41:749–59.

18
[15] Zugates GT, Tedford NC, Zumbuehl A, Jhunjhunwala S, Kang CS, Griffith LG, et al. Gene delivery properties of end-modified poly(beta-amino ester)s. Bioconjugate Chemistry 2007;18:1887–96.
[16] Lynn DM, Langer R. Degradable Poly ( -amino esters ): Synthesis , Characterization , and Self-Assembly with Plasmid DNA 2000:10761–8.
[17] Little SR, Lynn DM, Ge Q, Anderson DG, Puram SV, Chen J, et al. Poly-beta amino ester-containing microparticles enhance the activity of nonviral genetic vaccines. PNAS 2004;101:9534–9.
[18] Huang Y, Zugates GT, Peng W, Holtz D, Dunton C, Green JJ, et al. Nanoparticle-Delivered Suicide Gene Therapy Effectively Reduces Ovarian Tumor Burden in Mice. Cancer Research 2010;69:6184–91.
[19] Kamat CD, Shmueli RB, Connis N, Rudin CM, Green JJ, Hann CL. Poly(beta-aminoester) nanoparticle-delivery of p53 has activity against small cell lung cancer in vitro and in vivo. Molecular Cancer Therapeutics 2013.
[20] Sunshine JC, Sunshine SB, Bhutto I, Handa JT, Green JJ. Poly(β-amino ester)-nanoparticle mediated transfection of retinal pigment epithelial cells in vitro and in vivo. PloS One 2012;7:e37543.
[21] Tzeng SY, Hung BP, Grayson WL, Green JJ. Cystamine-terminated poly(beta-amino ester)s for siRNA delivery to human mesenchymal stem cells and enhancement of osteogenic differentiation. Biomaterials 2012;33:8142–51.
[22] Jere D, Xu C-X, Arote R, Yun C-H, Cho M-H, Cho C-S. Poly(beta-amino ester) as a carrier for si/shRNA delivery in lung cancer cells. Biomaterials 2008;29:2535–47.
[23] Green JJ, Zhou BY, Mitalipova MM, Beard C, Langer R, Jaenisch R, et al. Nanoparticles for Gene Transfer to Human Embryonic Stem Cell Colonies. Nano Lett 2008;8:3126–30.
[24] Yang F, Cho S-W, Son SM, Bogatyrev SR, Singh D, Green JJ, et al. Genetic engineering of human stem cells for enhanced angiogenesis using biodegradable polymeric nanoparticles. PNAS 2010;107:3317–22.
[25] Montserrat N, Garreta E, González F, Gutiérrez J, Eguizábal C, Ramos V, et al. Simple Generation of Human Induced Pluripotent Stem Cells Using Poly-β-amino Esters As the Non-viral Gene Delivery System. Journal of Biological Chemistry 2011;286 :12417–28.
[26] Anderson DG, Akinc A, Hossain N, Langer R. Structure/property studies of polymeric gene delivery using a library of poly(beta-amino esters). Molecular Therapy 2005;11:426–34.
[27] Akinc A, Lynn DM, Anderson DG, Langer R. Parallel synthesis and biophysical characterization of a degradable polymer library for gene delivery. JACS 2003;125:5316–23.
[28] Lynn DM, Anderson DG, Putnam D, Langer R. Accelerated discovery of synthetic transfection vectors: parallel synthesis and screening of a degradable polymer library. JACS 2001;123:8155–6.
[29] Zugates GT, Peng W, Zumbuehl A, Jhunjhunwala S, Huang Y-H, Langer R, et al. Rapid Optimization of Gene Delivery by Parallel End-modification of Poly([beta]-amino ester)s. Mol Ther 2007;15:1306–12.

19
[30] Akinc A, Anderson DG, Lynn DM, Langer R. Synthesis of poly(beta-amino ester)s optimized for highly effective gene delivery. Bioconjugate Chemistry 2003;14:979–88.
[31] Read ML, Singh S, Ahmed Z, Stevenson M, Briggs SS, Oupicky D, et al. A versatile reducible polycation-based system for efficient delivery of a broad range of nucleic acids. Nucleic Acids Research 2005;33 :e86–e86.
[32] Stevenson M, Ramos-Perez V, Singh S, Soliman M, Preece JA, Briggs SS, et al. Delivery of siRNA mediated by histidine-containing reducible polycations. J Controlled Release 2008;130:46–56.
[33] Abedini F, Hosseinkhani H, Ismail M, Domb AJ, Omar AR, Chong PP, et al. Cationized dextran nanoparticle-encapsulated CXCR4-siRNA enhanced correlation between CXCR4 expression and serum alkaline phosphatase in a mouse model of colorectal cancer. International Journal of Nanomedicine 2012;7:4159–68.
[34] Hosseinkhani H, Azzam T, Tabata Y, Domb AJ. Dextran-spermine polycation: an efficient nonviral vector for in vitro and in vivo gene transfection. Gene Ther 2004;11:194–203.
[35] Sunshine JC, Akanda MI, Li D, Kozielski KL, Green JJ. Effects of Base Polymer Hydrophobicity and End-Group Modification on Polymeric Gene Delivery. Biomacromolecules 2011;12:3592–600.
[36] Varkouhi AK, Scholte M, Storm G, Haisma HJ. Endosomal escape pathways for delivery of biologicals. J Controlled Release 2011;151:220–8.
[37] Behr J. The Proton Sponge: a Trick to Enter Cells the Viruses Did Not Exploit. Chimia 1997;2:34–6.
[38] Prabha S, Zhou W-Z, Panyam J, Labhasetwar V. Size-dependency of nanoparticle-mediated gene transfection: studies with fractionated nanoparticles. Int J Pharm 2002;244:105–15.
[39] Rejman J, Oberle V, Zuhorn IS, Hoekstra D. Size-dependent internalization of particles via the pathways of clathrin- and caveolae-mediated endocytosis. . Biochem J 2004;377:159–69.
[40] Ulasov AV, Khramtsov YV, Trusov GA, Rosenkranz AA, Sverdlov ED, Sobolev AS. Properties of PEI-based Polyplex Nanoparticles That Correlate With Their Transfection Efficacy. Mol Ther 2011;19:103–12.
[41] Hillaireau H, Couvreur P. Nanocarriers’ entry into the cell: relevance to drug delivery. Cell Mol Life Sci 2009;66:2873–2896 LA – English.
[42] Kunath K, von Harpe A, Fischer D, Petersen H, Bickel U, Voigt K, et al. Low-molecular-weight polyethylenimine as a non-viral vector for DNA delivery: comparison of physicochemical properties, transfection efficiency and in vivo distribution with high-molecular-weight polyethylenimine. J Controlled Release 2003;89:113–25.
[43] Yue Y, Wu C. Progress and perspectives in developing polymeric vectors for in vitro gene delivery. Biomaterials Science 2013;1:152–70.
[44] Fischer D, Li Y, Ahlemeyer B, Krieglstein J, Kissel T. In vitro cytotoxicity testing of polycations: influence of polymer structure on cell viability and hemolysis. Biomaterials 2003;24:1121–31.

20
[45] Lv H, Zhang S, Wang B, Cui S, Yan J. Toxicity of cationic lipids and cationic polymers in gene delivery. J Controlled Release 2006;114:100–9.
[46] Alexander C. Convergence of synthetic and natural polymers: next generation nanomedicines? Nanomedicine (London, England) 2008;3:749–51.
[47] Khan W, Hosseinkhani H, Ickowicz D, Hong P-D, Yu D-S, Domb AJ. Polysaccharide gene transfection agents. Acta Biomater 2012;8:4224–32.
[48] Hosseinkhani H, Aoyama T, Yamamoto S, Ogawa O, Tabata Y. In Vitro Transfection of Plasmid DNA by Amine Derivatives of Gelatin Accompanied with Ultrasound Irradiation. Pharm Res 2002;19:1471–9.
[49] Tzeng SY, Higgins LJ, Pomper MG, Green JJ. Student award winner in the Ph.D. category for the 2013 society for biomaterials annual meeting and exposition, april 10–13, 2013, Boston, Massachusetts . J Biomed Mater Res Part A 2013;101A:1837–45.
[50] Sunshine J, Green JJ, Mahon KP, Yang F, Eltoukhy AA, Nguyen DN, et al. Small-Molecule End-Groups of Linear Polymer Determine Cell-type Gene-Delivery Efficacy. Adv Mater 2009;21:4947–51.
[51] Li G-Z, Randev RK, Soeriyadi AH, Rees G, Boyer C, Tong Z, et al. Investigation into thiol-(meth)acrylate Michael addition reactions using amine and phosphine catalysts. Polym Chem 2010;1:1196–204.
[52] Mather BD, Viswanathan K, Miller KM, Long TE. Michael addition reactions in macromolecular design for emerging technologies. Prog Polym Sci 2006;31:487–531.
[53] Amini R, Hosseinkhani H, Jalilian FA. Engineered Smart Biomaterials for Gene Delivery. Gene Therapy and Molecular Biology 2012;14:72–86.
[54] Vazquez E, Roldán M, Diez-Gil C, Unzueta U, Domingo-Espín J, Cedano J, et al. Protein nanodisk assembling and intracellular trafficking powered by an arginine-rich (R9) peptide. Nanotechnology Biology & Medicine 2010;5:259–68.
[55] Kim HH, Lee WS, Yang JM, Shin S. Basic peptide system for efficient delivery of foreign genes. Biochimica Et Biophysica Acta (BBA) - Molecular Cell Research 2003;1640:129–36.
[56] Mitchell DJ, Steinman L, Kim DT, Fathman CG, Rothbard JB. Polyarginine enters cells more efficiently than other polycationic homopolymers. The Journal of Peptide Research 2000;56:318–25.
[57] Kawamura KS, Sung M, Bolewska-Pedyczak E, Gariépy J. Probing the Impact of Valency on the Routing of Arginine-Rich Peptides into Eukaryotic Cells†. Biochemistry 2006;45:1116–27.
[58] Futaki S, Ohashi W, Suzuki T, Niwa M, Tanaka S, Ueda K, et al. Stearylated Arginine-Rich Peptides: A New Class of Transfection Systems. Bioconjugate Chem 2001;12:1005–11.
[59] Choi JS, Nam K, Park J, Kim J-B, Lee J-K, Park J. Enhanced transfection efficiency of PAMAM dendrimer by surface modification with l-arginine. J Controlled Release 2004;99:445–56.

21
[60] Okuda T, Sugiyama A, Niidome T, Aoyagi H. Characters of dendritic poly(l-lysine) analogues with the terminal lysines replaced with arginines and histidines as gene carriers in vitro. Biomaterials 2004;25:537–44.
[61] Shmueli RB, Sunshine JC, Xu Z, Duh EJ, Green JJ. Gene delivery nanoparticles specific for human microvasculature and macrovasculature. Nanomed Nanotechnol Biol Med 2012;8:1200–7.
[62] Tzeng SY, Guerrero-Cázares H, Martinez EE, Sunshine JC, Quiñones-Hinojosa A, Green JJ. Non-viral gene delivery nanoparticles based on Poly(β-amino esters) for treatment of glioblastoma. Biomaterials 2011;32:5402–10.
[63] Felgner PL, Tsai YJ, Sukhu L, Wheeler CJ, Manthorpe M, Marshall J, et al. Improved Cationic Lipid Formulations for In Vivo Gene Therapy. Ann NY Acad Sci 1995;772:126–39.
[64] Felgner JH, Kumar R, Sridhar CN, Wheeler CJ, Tsai YJ, Border R, et al. Enhanced gene delivery and mechanism studies with a novel series of cationic lipid formulations. Journal of Biological Chemistry 1994;269 :2550–61.
[65] Cho EC, Zhang Q, Xia Y. The effect of sedimentation and diffusion on cellular uptake of gold nanoparticles. Nat Nano 2011;6:385–91.
[66] Zuber G, Dauty E, Nothisen M, Belguise P, Behr J-P. Towards synthetic viruses. Adv Drug Delivery Rev 2001;52:245–53.
[67] De Laporte L, Cruz Rea J, Shea LD. Design of modular non-viral gene therapy vectors. Biomaterials 2006;27:947–54.
[68] Won Y-W, Lim KS, Kim Y-H. Intracellular organelle-targeted non-viral gene delivery systems. J Controlled Release 2011;152:99–109.
[69] Cherng J-Y, Lee Y-P, Lin C-H, Chang K-H, Chang W-Y, Shau M-D. The characteristics and transfection efficiency of PEI modified by biodegradable poly(β-amino ester). J Mater Sci: Mater Med 2010;21:1543–1551 LA – English.
[70] Hwang HS, Kang HC, Bae YH. Bioreducible Polymers As a Determining Factor for Polyplex Decomplexation Rate and Transfection. Biomacromolecules 2012;14:548–56.
[71] Zhang Y, Yang M, Park J-H, Singelyn J, Ma H, Sailor MJ, et al. A Surface-Charge Study on Cellular-Uptake Behavior of F3-Peptide-Conjugated Iron Oxide Nanoparticles. Small 2009;5:1990–6.
[72] Bondar OV, Saifullina DV, Shakhmaeva II, Mavlyutova II, Abdullin TI. Monitoring of the Zeta Potential of Human Cells upon Reduction in Their Viability and Interaction with Polymers. Acta Naturae 2012;4:78–81.
[73] Ruponen M, Rönkkö S, Honkakoski P, Pelkonen J, Tammi M, Urtti A. Extracellular Glycosaminoglycans Modify Cellular Trafficking of Lipoplexes and Polyplexes. Journal of Biological Chemistry 2001;276 :33875–80.
[74] Ruponen M, Honkakoski P, Tammi M, Urtti A. Cell-surface glycosaminoglycans inhibit cation-mediated gene transfer. The Journal of Gene Medicine 2004;6:405–14.

22

23

24

25

26

27

28

29

30
Poly(β-amino ester) end-modifying moiety
B3 NH2-CH2-(CH2)2-CH(CH3)-CH2-NH2
R3C-C32-CR3 SH-Cys-Arg-Arg-Arg-CONH2 · 4HCl
K3C-C32-CK3 SH-Cys-Lys-Lys-Lys-CONH2 · 4HCl
H3C-C32-CH3 SH-Cys-His-His-His-CONH2 · 4HCl

31