Homework Problem Set 7 Solutions - Illinois State … 380.37...Atom x y z A –0.50 –0.23 0.00 B...
Transcript of Homework Problem Set 7 Solutions - Illinois State … 380.37...Atom x y z A –0.50 –0.23 0.00 B...

Chemistry 380.37 Dr. Jean M. Standard
Homework Problem Set 7 Solutions
1. Which molecule would be expected to have the higher diffusion coefficient in the gas phase, CH4 or H2? Explain. The diffusion coefficient is related to the average speed vave and mean free path λ of the molecules,
D = 12λvave .
At a given temperature, CH4 and H2 will have the same average kinetic energy, E, given classically by the equation
E = 12mvave
2 .
Solving for the average speed yields the equation,
vave = 2Em
!
"#
$
%&1/2
,
where m is the mass of the molecule. Here we see that the average speed is inversely proportional to mass, so H2 will have the higher average speed and the heavier CH4 molecule will have the lower average speed and therefore the smaller diffusion coefficient. H2 will have a larger average speed and a larger diffusion coefficient.
2. In the liquid phase, which molecule would be expected to have the higher diffusion coefficient,
methanol or octanol? Explain.
By a similar argument to that given in Problem 1, octanol, the heavier and therefore slower molecule, is expected to have a lower diffusion coefficient. Methanol, the lighter and faster molecule relative to octanol, is expected to have a higher diffusion coefficient.

2
3. The gas phase diffusion coefficient of methane is 1.78×10–5 m2s–1. Assuming a mean free path of 80 nm, calculate the average speed of methane molecules in m/s.
The diffusion coefficient is related to the average speed vave and mean free path λ by the equation
D = 12λvave .
Solving for the average speed yields
vave = 2Dλ
.
Substituting,
vave = 2Dλ
= 2 1.78×10−5 m2s−1( )
80×10−9 mvave = 445 m/s.
4. For a molecule undergoing collisions and moving through a liquid solution, the average distance squared
from the molecule's initial position, r2 , is linearly related to the time,
r2 = Dt,
where D is the diffusion coefficient. In contrast, for a molecule that is allowed to freely translate in space with no collisions, determine how the distance squared varies with time. Assume classical behavior and compare with the diffusional behavior in liquid solution. Classically, assuming that the particle starts at the origin, the distance from the origin r as a function of time is related directly to the velocity v,
r = v t. Squaring both sides yields
r2 = v2 t2 . So we see that for free translation with no collisions, the squared position r2 varies directly as t2 , while for a particle undergoing collisions and diffusional motion, the average squared position r2 varies linearly with
time. This means that for a particle undergoing free translational motion, the particle moves away from the origin at a much faster rate than for one undergoing diffusional motion.

3
5. Consider a bond involving atoms A and B represented using a harmonic force field of the form
€
U = 12 ks,AB rAB − rAB,eq( )2
, where
€
rAB is the instantaneous bond length and
€
rAB,eq is the equilibrium bond length. The equilibrium A-B bond length is 1.00 Å, and the stretching force constant is 500 kcal mol–1 Å–2. The atoms are arranged so that they have the cartesian coordinates given below (in Å).
Atom x y z
A –0.50 –0.23 0.00 B 0.37 0.54 0.00
a.) What is the instantaneous A-B bond length?
€
rAB = xA − xB( )2 + yA − yB( )2 + zA − zB( )2[ ]1/ 2
= −0.50 − 0.37 Å( )2+ −0.23− 0.54 Å( )2
+ 0 − 0( )2# $ %
& ' (
1/ 2
rAB = 1.16 Å .
b.) What is the potential energy of the system in units of kcal mol–1?
€
U = 12 ks,AB rAB − rAB,eq( )2
= 12 500 kcalmol−1Å−2( ) 1.16−1.00 Å( )2
U = 6.56 kcalmol−1.
c.) Determine the x-, y-, and z-components of the force on atom A.
The force on atom A is determined as the partial derivative of the force field with respect to the cartesian coordinates. For the force on atom A in the x direction,
€
FA,x = − ∂U∂ xA
$
% &
'
( )
= − ∂U∂ rAB
$
% &
'
( ) ∂ rAB∂ xA
$
% &
'
( )
FA,x = − ks,AB rAB − rAB ,eq( ) ∂ rAB∂ xA
$
% &
'
( ) .

4
5 c.) continued
To complete the derivation of the force, the derivative of the bond length
€
rAB with respect to the cartesian coordinate
€
xA must be determined.
€
∂ rAB∂ xA
#
$ %
&
' ( = 1
2 xA − xB( )2 + yA − yB( )2 + zA − zB( )2[ ]−1/ 22( ) xA − xB( )
∂ rAB∂ xA
#
$ %
&
' ( =
xA − xBrAB
.
Substituting,
€
FA,x = − ks,AB rAB − rAB ,eq( ) xA − xBrAB
#
$ %
&
' (
= − 500 kcalmol−1Å−2( ) 1.16−1.00 Å( ) −0.50− 0.37 Å1.16 Å
#
$ %
&
' (
FA,x = 60.6 kcalmol−1Å−1.
Similarly, the y-component of the force can be calculated,
€
FA,y = − ks,AB rAB − rAB ,eq( ) yA − yBrAB
#
$ %
&
' (
= − 500 kcalmol−1Å−2( ) 1.16−1.00 Å( ) −0.23− 0.54 Å1.16 Å
#
$ %
&
' (
FA,y = 53.6 kcalmol−1Å−1.
Finally, the z-component is calculated,
€
FA,z = − ks,AB rAB − rAB ,eq( ) zA − zBrAB
#
$ %
&
' (
= − 500 kcalmol−1Å−2( ) 1.16−1.00 Å( ) 0.0− 0.0 Å1.16 Å
#
$ %
&
' (
FA,z = 0.
d.) Determine the x-, y-, and z-components of the force on atom B.
The force on atom B is determined as the partial derivative of the force field with respect to the cartesian coordinates. As in part (c) above, the x component of the force on atom B is
€
FB,x = − ∂U∂ xB
$
% &
'
( )
= − ∂U∂ rAB
$
% &
'
( ) ∂ rAB∂ xB
$
% &
'
( )
FB,x = − ks,AB rAB − rAB ,eq( ) ∂ rAB∂ xB
$
% &
'
( ) .

5
5 d.) continued To complete the derivation of the force, the derivative of the bond length
€
rAB with respect to the cartesian coordinate
€
xB must be determined.
€
∂ rAB∂ xB
#
$ %
&
' ( = 1
2 xA − xB( )2 + yA − yB( )2 + zA − zB( )2[ ]−1/ 2−2( ) xA − xB( )
∂ rAB∂ xB
#
$ %
&
' ( = −
xA − xBrAB
.
Substituting,
€
FB,x = ks,AB rAB − rAB,eq( ) xA − xBrAB
#
$ %
&
' (
= 500 kcalmol−1Å−2( ) 1.16−1.00 Å( ) −0.50− 0.37 Å1.16 Å
#
$ %
&
' (
FB,x = − 60.6 kcalmol−1Å−1.
Similarly, the y-component of the force can be calculated,
€
FB,y = ks,AB rAB − rAB,eq( ) yA − yBrAB
#
$ %
&
' (
= 500 kcalmol−1Å−2( ) 1.16−1.00 Å( ) −0.23− 0.54 Å1.16 Å
#
$ %
&
' (
FB,y = − 53.6 kcalmol−1Å−1.
Finally, the z-component is calculated,
€
FB,z = ks,AB rAB − rAB,eq( ) zA − zBrAB
#
$ %
&
' (
= 500 kcalmol−1Å−2( ) 1.16−1.00 Å( ) 0.0− 0.0 Å1.16 Å
#
$ %
&
' (
FB,z = 0.

6
5. continued
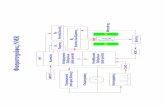
e.) Are the atoms moving closer together or farther apart? The positions of the two atoms are shown in the figure below (note that the force vectors are not drawn to scale). The force on atom A is in the positive x and positive y direction (along the bond). The force on atom B is in the negative x and negative y direction (also along the bond). Thus, the atoms are moving closer together.

7
6. Consider the simple one-dimensional harmonic potential as a representation of the motion of a bond in a
molecular system,
€
U x( ) = 12 k x
2.
The coordinate x represents the bond displacement,
€
x = r − req . Assume that the force constant k for the bond is 720 kcal mol–1 Å–2. The reduced mass is 12.0 g/mol. The bond displacement coordinate has an initial value of 0 Å. The initial velocity is 8400 m/s.
a.) What is the potential energy?
€
U = 12 kx
2
= 12 720 kcalmol−1Å−2( ) 0( )2
U = 0.
b.) What is the kinetic energy of the system?
€
T = 12 mv
2
= 12 0.012 kg mol−1( ) 8400 ms−1( )2
= 4.23×105 J/mol
T = 1.01×105 cal/mol (or 101 kcal/mol).
c.) What is the total energy of the system?
€
E = T + U= 101 + 0 kcal/mol
E = 101 kcal/mol.

8
7. Consider the potential energy function studied in problem 6 with the same parameters. Since the potential energy function described in problem 6 is harmonic, the bond undergoes simple harmonic motion as a function of time. The exact analytical solutions of Newton's equations for a harmonic oscillator with an initial position of zero and an initial velocity v(0) are
€
x(t) = v(0)ω
sin ω t( )
v(t) = v(0) cos ω t( ) .
The parameter ω is the angular velocity and is defined by the relation
€
ω = km
, where m is the mass. The angular velocity ω is related to the harmonic frequency of oscillation
€
ν o ,
€
ν o = ω2π
.
a.) Using the same parameters as given in problem 6, determine the harmonic frequency of vibration for
this system.
For simple harmonic motion, the harmonic frequency of vibration
€
ν o is
€
ν o = ω2π
= 12π
km
= 12π
720 kcalmol−1Å−2
0.012 kg mol−1
&
' (
)
* +
4.184 kJ1kcal
&
' (
)
* +
1000 J1kJ
&
' (
)
* +
1Å10−10 m
&
' (
)
* +
2
ν o = 2.52×1013 s−1.
b.) Using the analytic solution to Newton's equations for the harmonic oscillator, plot the bond
displacement coordinate x as a function of time. For simple harmonic motion and the provided initial conditions, the analytic expression for the position is
€
x(t) = v(0)ω
sin ω t( ) .
Using the harmonic vibrational frequency
€
ν o calculated in part (a), the angular frequency ω can be determined,
€
ω = 2π ν o
= 2π 2.52×1013 s−1( )ω = 1.58×1014 s−1.

9
7 b.) continued
The initial velocity is
€
v 0( ) = 8400 m/s . Thus, the position as a function of time is given by the equation
€
x t( ) = v(0)ω
sin ω t( )
= 8400 m/s1.58×1014 s−1
%
& '
(
) * sin 1.58×1014 s−1 t( )
= 5.31×10−11 m( ) sin 1.58×1014 s−1 t( )x t( ) = 0.531Å( ) sin 1.58×1014 s−1 t( ) .
A plot of this function versus time is shown in the figure below.
c.) Using the analytic solution to Newton's equations for the harmonic oscillator, plot the velocity as a function of time.
The velocity is the time derivative of the position,
€
v t( ) = ˙ x t( ) = ddt
x t( )
= ddt
v(0)ω
sin ω t( )#
$ % &
' (
v t( ) = v 0( ) cos ω t( ) .

10
7 c.) continued
Substituting the specific parameters for this problem, the expression for the velocity becomes
€
v t( ) = 8400 m/s( ) cos 1.58×1014 s−1 t( ), with the velocity in m/s and the frequency in s–1. A plot of the velocity as a function of time is shown in the figure below.

11
8. Again consider the simple one-dimensional potential used in problems 6 and 7. Assume that the force constant k is 720 kcal mol–1 Å–2. The reduced mass is 12.0 g/mol. The initial position is 0 Å and the initial velocity is 8400 m/s. a.) Using the Verlet leapfrog algorithm, carry out two time steps to determine the position and velocity
of the particle at times t1 and t2. Use a step size of 1 femtosecond.
The Verlet leapfrog algorithm for one particle in one dimension is given by
€
x tn+1( ) = x tn( ) + hvx tn+1/ 2( )
vx tn+1/ 2( ) = vx tn−1/ 2( ) + hmFx tn( ) .
The force
€
Fx is obtained from the force field,
€
Fx = − dUdx
Fx = − kx .
Subsituting, the velocity equation becomes
€
vx t n+1/ 2( ) = vx t n−1/ 2( ) − hkmx tn( ).
For n = 0, the Verlet leapfrog equations are given by
€
vx t1/ 2( ) = vx t−1/ 2( ) − hkmx t0( )
x t1( ) = x t0( ) + h vx t1/ 2( ) .
The time step is
€
h = 10−15 s . Using the initial conditions
€
x t0( ) = x 0( ) = 0 and
€
vx t−1/2( ) = vx 0( ) = 8400 m/s , the velocity equations is
€
vx t1/ 2( ) = vx t−1/ 2( ) − hkmx t0( )
= 8400 m/s − hkm
0( )
vx t1/ 2( ) = 8400 m/s .
The position equation then becomes
€
x t1( ) = x t0( ) + h vx t1/ 2( )= 0 + 10−15 s( ) 8400 m/s( )
x t1( ) = 8.40× 10−12 m.

12
8 a.) continued For the next time step, with n = 1, the velocity equation becomes
€
vx t 3/ 2( ) = vx t1 /2( ) − hkmx t1( ).
In order to evaluate this equation, it is helpful to convert the force constant into SI units,
€
k = 760 kcalmol−1Å−2( ) 4.184 kJ1kcal
#
$ %
&
' (
1000 J1kJ
#
$ %
&
' (
1Å10−10 m
#
$ % %
&
' ( (
2
k = 3.01×1026 J mol−1m−2.
Substituting the force constant into the velocity equation yields
€
vx t3/ 2( ) = vx t1/ 2( ) − hkmx t1( )
= 8400 m/s −10−15 s( ) 3.01× 1026 J mol−1m−2( ) 8.40× 10−12 m( )
0.012 kg mol−1( )vx t3/ 2( ) = 8189 m/s .
For the time step with n = 1 the position equation becomes
€
x t2( ) = x t1( ) + h vx t3/ 2( ) . Substituting,
€
x t2( ) = 8.40×10−12 m + 10−15 s( ) 8189 m/s( )
x t2( ) = 1.66×10−11 m.
Finally, for the final time step, with n = 2, the velocity equation becomes
€
vx t5 / 2( ) = vx t3/ 2( ) − hkmx t2( ) .
Using the force constant in the velocity equation yields
€
vx t5 / 2( ) = 8189 m/s−10−15 s( ) 3.01×1026 J mol−1m−2( ) 1.66×10−11 m( )
0.012 kg mol−1( )vx t5 / 2( ) = 7773 m/s .
Averaging the first pair of velocities, the equations are
€
vx t0( ) = 12 vx t1/ 2( ) + vx t−1/ 2( )[ ]
= 12 8400 m/s + 8400 m/s[ ]
vx t0( ) = 8400 m/s .

13
8 a.) continued Averaging the second pair of velocities yields
€
vx t1( ) = 12 vx t3/ 2( ) + vx t1/ 2( )[ ]
= 12 8189 m/s + 8400 m/s[ ]
vx t1( ) = 8295m/s .
Averaging the final pair of velocities yields
€
vx t2( ) = 12 vx t5 / 2( ) + vx t3/ 2( )[ ]
= 12 7773m/s + 8189 m/s[ ]
vx t2( ) = 7981m/s .
These results are summarized in the table below.
n
€
tn
(s)
€
x tn( )
(m)
€
vx t n( )
(m/s)
0 0 0 8400
1
€
1×10−15
€
8.40× 10−12 8295
2
€
2× 10−15
€
1.66 ×10−11 7981
b.) Compare your results from part (a) to the exact solution obtained in problem 7. The equations for the exact analytic solutions for the position and velocity are given in problem 7. The position is
€
x t( ) = 0.531 Å( ) sin 1.58×1014 s−1 t( ) ,
with time in seconds and position in angstroms. The velocity is
€
v t( ) = 8400 m/s( ) cos 1.58×1014 s−1 t( ) ,
with the velocity in m/s and time in seconds.

14
8 b.) continued The results for the analytic solution, using the same time points as in part (b), yields the results presented in the table below.
n
€
tn
(s)
€
x tn( )
(m)
€
vx t n( )
(m/s)
0 0 0 8400
1
€
1×10−15
€
8.363×10−12 8295
2
€
2× 10−15
€
1.652 ×10−11 7982
We can see from these results that the leapfrog algorithm predicts the velocities with little error compared to the analytic solution. The percent errors for the n = 0, 1, and 2 time steps are 0%, 0%, and 0.01%, respectively. The leapfrog algorithm also does a reasonable job in predicting the position of the particle compared to the analytic solution. The errors are somewhat larger than those for the velocity, with errors of 0%, 0.4%, and 0.6%, respectively.

15
9. The Maxwell-Boltzmann distribution for speeds is
€
F (v) = 4π v2 m
2π kBT#
$ %
&
' (
3/ 2
exp −mv2
2kBT#
$ %
&
' (
.
In this equation, m is the mass of a particle,
€
kB is the Boltzmann constant, T is temperature, and v is the speed. The speed v is defined to be the magnitude of the velocity vector,
€
v = vx2 + vy
2 + vz2[ ]1/ 2
,
where
€
vx ,
€
vy , and
€
vz are the components of the velocity vector. The most probable speed
€
vmp corresponding to the maximum in the distribution is given by the equation
€
vmp = 2kBTm
" # $
% & '
1/ 2.
a.) Plot the Maxwell-Boltzmann distribution function
€
F (v) (y-axis) versus v (x-axis) for a collection of atoms at 300 K. Assume that one mole of atoms weighs 12.0 g.
b.) Using the same mass as in part (a), plot the Maxwell-Boltzmann distribution function
€
F (v) versus v for a collection of atoms at 1000 K. Discuss the effect of temperature on the distribution of atomic speeds. Both questions (a) and (b) are answered below. The Maxwell-Boltzmann distribution function for the atoms at 300 K is given by
€
F v( ) = 4π v20.012 kg/mol( ) 1mol
6.02217× 1023$ % &
' ( )
2π 1.38062× 10-23 JK−1( ) 300 K( )
+
,
- - - -
.
/
0 0 0 0
3/ 2
× exp −0.012 kg/mol( ) 1mol
6.02217× 1023$ % &
' ( ) v2
2 1.38062× 10-23 JK−1( ) 300 K( )
1
2 3 3
4 3 3
5
6 3 3
7 3 3
€
F v( ) = 8.41956× 10−9 v2( ) exp −2.40549× 10−6 v2{ }.

16
9a, b.) continued The velocity distribution function for the atoms at 1000 K is given by
€
F v( ) = 4π v2 0.012 kg/mol( ) 1mol
6.02217× 1023$ % &
' ( )
2π 1.38062× 10-23 JK−1( ) 1000 K( )
+
,
- - - -
.
/
0 0 0 0
3/ 2
× exp −0.012 kg/mol( ) 1mol
6.02217× 1023$ % &
' ( ) v2
2 1.38062× 10-23 JK−1( ) 1000 K( )
1
2 3 3
4 3 3
5
6 3 3
7 3 3
€
F v( ) = 1.38347× 10−9 v2( ) exp −7.21646× 10−7 v2{ }.
Note that the units of the exponent cancel out so that it is unitless, while the units of the terms in front of the exponent are sm–1.
Plots of the Maxwell-Boltzmann distribution of speeds at 300 and 1000 K are shown on a single graph below.
As can be seen from the graph, the maximum (or most probable) speed increases as the temperature increases. In addition, the entire distribution shifts to larger speeds as the temperature increases.

17
9. continued c.) Calculate the most probable speed for atoms weighing 12.0 g/mol at 300 K and 1000 K. Express your
answers in m/s. The most probable speed
€
vmp is
€
vmp = 2kBTm
" # $
% & '
1/ 2.
At 300 K, the most probable speed is therefore
€
vmp = 2 1.38062× 10−23 JK−1( ) 300 K( )
0.012 kg/mol( ) 1mol /6.02217× 1023( )$
%
& &
'
(
) )
1/ 2
vmp = 645 m/s .
At 1000 K, the most probable speed is
€
vmp = 2 1.38062× 10−23 JK−1( ) 1000 K( )
0.012 kg/mol( ) 1mol /6.02217× 1023( )$
%
& &
'
(
) )
1/ 2
vmp = 1180 m/s .
d.) From the most probable speeds calculated in part (c), determine the most probable kinetic energy of
one mole of atoms at 300 K and 1000 K. Express your answers in J/mol. The most probable kinetic energy
€
KEmp is given by
€
KEmp = 12 m vmp
2 .
At 300 K, the most probable kinetic energy is
€
KEmp = 12 0.012 kg/mol( ) 645m/s( )2
KEmp = 2490 J/mol.
At 1000 K, the most probable kinetic energy is
€
KEmp = 12 0.012 kg/mol( ) 1180 m/s( )2
KEmp = 8310 J/mol.

18
10. The Equipartition Theorem states that each degree of freedom contributes
€
12 kBT to the internal energy
of a particle (or
€
12 RT to the internal energy of one mole of particles).
a.) Use the equipartition theorem to calculate the internal energy of a mole of atoms at 300 K. Express
your answer in J/mol. Since an atom has only three translational degrees of freedom (and no vibrational or rotational degrees of freedom), the internal energy U for one mole of atoms at 300 K is
€
U = 32 RT
= 32 8.314 J/molK( ) 300 K( )
U = 3740 J/mol.
b.) Repeat the calculation of the internal energy for a mole of atoms at 1000 K. Express your answer in
J/mol. The internal energy U for one mole of atoms at 1000 K is
€
U = 32 RT
= 32 8.314 J/molK( ) 1000 K( )
U = 12500 J/mol.
c.) Compare your results from parts (a) and (b) to the kinetic energy determined in part 9(d). Are the
values similar? The internal energy values are similar in magnitude to the most probably kinetic energy values, 3740 vs. 2490 J/mol at 300 K, and 12500 vs. 8310 J/mol at 1000 K. The internal energy values are a little lower than the most probable kinetic energy. This is because the distribution of speeds is skewed to higher speeds; therefore, the average speed is greater than the most probable speed. The Equipartition Theorem provides a measure of the average kinetic energy of the system rather than the most probable kinetic energy; thus, the internal energy computed from the Equipartition Theorem is larger than the most probable kinetic energy.