Fundamentals of Analytical Chemistry - Cameron …cameron.edu/~keithv/theory/AC_10.pdf ·...
Click here to load reader
Transcript of Fundamentals of Analytical Chemistry - Cameron …cameron.edu/~keithv/theory/AC_10.pdf ·...

1
Fundamentals of Fundamentals of
Analytical ChemistryAnalytical Chemistry
Chapter 10Chapter 10
Effect of Electrolytes on Chemical Effect of Electrolytes on Chemical
EquilibriaEquilibria
HomeworkHomework
�� 7, 8, 107, 8, 10--12, 1412, 14
�� Test 1 will be given when we finish Test 1 will be given when we finish
Chapter 10!Chapter 10!
EquilibriumEquilibrium
�� For the reaction For the reaction AgCl(sAgCl(s) ) �� AgAg++ + + ClCl--
�� What is the effect when addingWhat is the effect when adding
�� AgNOAgNO33
�� NaClNaCl
�� NaNONaNO33
Concentration Equilibrium ConstantConcentration Equilibrium Constant
�� It can be shown that the equilibrium It can be shown that the equilibrium constant (as we know it) is a limiting valueconstant (as we know it) is a limiting value�� Minimum value for the systemMinimum value for the system
�� Value will increase as the number and/or Value will increase as the number and/or charge of ions in solution increasescharge of ions in solution increases
�� Up to about 0.1M effect is independent of the Up to about 0.1M effect is independent of the type of iontype of ion�� Above this value we get ionAbove this value we get ion--specific variationsspecific variations
�� Define KDefine K’’ = [= [AgAg++][Cl][Cl--]]�� Note that KNote that K’’ changes with the ionic strength changes with the ionic strength of the solutionof the solution
Ionic StrengthIonic Strength
�� Solution parameterSolution parameter
�� Accounts for both concentration and Accounts for both concentration and
charge of ions in solutioncharge of ions in solution
��
�� Allows us to predict the effect on Allows us to predict the effect on
individual ions IF individual ions IF µµ is less than about 0.1is less than about 0.1
∑= 2
2
1iiZCµ
Ionic StrengthIonic Strength
�� Note that for a solution consisting of only Note that for a solution consisting of only
singlysingly--charged ions, the ionic strength will charged ions, the ionic strength will
equal the concentration of the equal the concentration of the salt(ssalt(s))
�� For solutions with higher charged ions, the For solutions with higher charged ions, the
ionic strength will always be greater than the ionic strength will always be greater than the
concentration of the salts!concentration of the salts!
�� The ionic strength has no effect on The ionic strength has no effect on
uncharged speciesuncharged species

2
Activity CoefficientActivity Coefficient
�� Effective concentration of ionsEffective concentration of ions
�� Applies to individual ions in solutionApplies to individual ions in solution
�� Maximum value will be the equilibrium concentration Maximum value will be the equilibrium concentration
of the ionof the ion
�� Decrease from there as a function ofDecrease from there as a function of
�� ItIt’’s charges charge
�� ItIt’’s s ‘‘hydrated radiushydrated radius’’
�� The ionic strength of the solutionThe ionic strength of the solution
�� ActivityActivity
�� aaxx = [X]= [X]γγxx�� γγxx = activity coefficient= activity coefficient
Activity CoefficientActivity Coefficient
�� Maximum value = 1Maximum value = 1
�� Only when Only when µµ = 0= 0
�� Essentially = 1 when Essentially = 1 when µµ < 10< 10--55
�� Redefine KRedefine K
�� For dissociation of For dissociation of AgClAgCl, K = , K = aaAgAgaaClCl�� Substitute activities for concentrationsSubstitute activities for concentrations
�� Thermodynamic equilibrium constantThermodynamic equilibrium constant
�� Tabulated values are K, not KTabulated values are K, not K’’
�� NOT a function of ionic strengthNOT a function of ionic strength
�� KK’’ = concentration equilibrium constant= concentration equilibrium constant
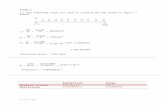
Relationship of K and KRelationship of K and K’’
−+
−+
−+
−+
=′∴
′=
=
•=
=
+↔
−+
−+
−+
−+
ClAg
ClAg
ClAg
ClAg
KK
KClAgBut
ClAgK
ClAgK
aaK
ClAgsAgClFor
γγ
γγ
γγ
]][[
]][[
][][
)(
Calculating Calculating γγ
�� DebyeDebye--HuckelHuckel equationequation
��
�� γγxx = activity coefficient of X= activity coefficient of X
�� ZZxx = charge on X= charge on X
�� µµ = ionic strength = ionic strength of the solutionof the solution
�� ααxx = diameter of hydrated ion in nm= diameter of hydrated ion in nm
�� 0.51,0.51, 3.3 function of water at 253.3 function of water at 25OOCC
µα
µγ
x
x
x
Z
3.31
51.0log
2
+−=
Table 10Table 10--22
�� Hydrated radii of ionsHydrated radii of ions
�� Also contains calculated values for the Also contains calculated values for the
activity coefficient for many ions at the activity coefficient for many ions at the
indicated ionic strengthsindicated ionic strengths
�� If If µµ is equal to or close to one of the is equal to or close to one of the taulatedtaulated
values, then you can look up values, then you can look up γγ rather than rather than
calculate it!!!calculate it!!!
ProblemsProblems
�� We are interested in the concentration of We are interested in the concentration of
species, not the activityspecies, not the activity
�� Equilibrium data provided is for activities, Equilibrium data provided is for activities,
not concentrationsnot concentrations
�� MUST be able to relate the twoMUST be able to relate the two