Treatment for Glioblastoma with Carboplatin, Oral ... · PDF fileCentral JSM Neurosurgery and...
Transcript of Treatment for Glioblastoma with Carboplatin, Oral ... · PDF fileCentral JSM Neurosurgery and...

Central JSM Neurosurgery and Spine
Cite this article: Kanno H, Nakanowatari S (2014) Treatment for Glioblastoma with Carboplatin, Oral Etoposide and Interferon β (CEI): A Phase II Trial. JSM Neurosurg Spine 2(3): 1027.
*Corresponding authorHiroshi Kanno, Department of Neurosurgery, Yokohama City University School of Medicine, Japan, E-mail:
Submitted: 05 February 2014
Accepted: 03 March 2014
Published: 20 March 2014
Copyright© 2014 Kanno et al.
OPEN ACCESS
Keywords•Chemotherapy•Glioblastoma•Carboplatin•Etoposide•Interferonβ
Research Article
Treatment for Glioblastoma with Carboplatin, Oral Etoposide and Interferon β (CEI): A Phase II TrialHiroshi Kanno* and Satoshi Nakanowatari Department of Neurosurgery, Yokohama City University School of Medicine, Japan
Abstract
Gioblastoma is one of the most refractory tumors in central nervous system, and the prognosis remains poor. Temozolomide is a first-line agent for newly-diagnosed glioblastoma, but chemotherapy for recurrent or temozolomide-resistant glioblastoma has not been established. To determine whether chemotherapy with carboplatin, oral etoposide, and interferon β (CEI) prolongs survivals of patients bearing glioblastoma, chemotherapy with CEI was undergone for twenty patients with glioblastoma after surgery. This trial was an open-label, single-center phase II study. In all 20 enrolled patients, the median progression-free survival (PFS) was 10.5 months, and PFS at 6 and 12 months after CEI therapy were 85% and 45%, respectively. The median overall survival was 15.0 months, and 95% and 80% of the patients were alive at 6 and 12 months after CEI therapy, respectively. This regimen was well tolerated and could be one of the options for patients with glioblastoma.
INTRODUCTION Glioblastoma is a highly lethal tumor in central nervous
system. The prognosis is still poor, and the median overall survival of patients with glioblastoma is not over one and a half years at present. The standard therapy of a newly diagnosed glioblastoma usually consists of cytoreductive surgery followed by conventional radiotherapy concomitant with chemotherapy. Recently, temozolomide is globally recommended as a first-line agent for glioblastoma and has been used as standard chemotherapy in patients with glioblastoma [1], instead of nitrosourea agents such as CCNU, BCNU, and ACNU. Intravenous infusion of carboplatin or irinotecan and oral etoposide, are recommended for the second selective drug. In addition, agents having a cytostatic effect as retinoic acid, thalidomide, and tamoxifen are occasionally used [2].
We previously examined the chemosensitivities of malignant gliomas for four anticancer agents (ACNU, carboplatin, cisplatin, and etoposide) except for temozolomide by using a collagen gel matrix assay, and among the used therapeutic drugs, carboplatin was selected as an agent showing the highest sensitivity, and next etoposide was done [3]. There are many reports of chemotherapy with etoposide for high-grade gliomas [4-8], but etoposide is usually used with not oral administration but intravenous infusion. However, effectiveness of chemotherapy with oral etoposide for glioblastoma has been reported [9-11].
Carboplatin requires less hydration burden than cisplatin inducing renal dysfunction, and can be used at outpatient clinic. Interferon β is also used at the outpatient clinic and oral etoposide is taken at home. Since a patient with glioblastoma shows longer survival as higher quality of life (QOL) [12], longer living at home with higher QOL is better. Based on the chemosensitivities and preservation of high QOL, we employed carboplatin and oral etoposide, and additionally interferon β, and examined the outcome of globlastoma patients underwent chemotherapy with CEI (carboplatin and oral etoposide, and interferon β), and additionally discussed CEI therapy.
OBJECTS AND METHODS Objective
The objects of this study are 20 patients bearing glioblastoma underwent surgery, radiotherapy, and chemotherapy with carboplatin, oral etoposide, and β interferon at Yokohama City University Hospital during April in 2003 to March in 2008. The patients were 13 males and 7 females, and the beginning age of chemotherapy ranged from 22 to 78 years old. The all patients were finally diagnosed as glioblastoma, and the tumors were pathologically verified. The 15 patients had primary glioblastoma (de novo glioblastoma), and the remaining 5 patients had secondary glioblastoma to which low-grade astrocytoma malignantly transformed. The tumors were located at frontal

Central
Kanno et al. (2014)Email:
JSM Neurosurg Spine 2(3): 1027 (2014) 2/4
lobe, 5; temporal lobe, 8; parietal lobe, 2; temporo-occipital, 2; pineal region, 2; frontal to parietal lobe, 1. They underwent CEI therapy after surgery. Repeat times of CEI therapy were 3-6 times (Table 1).
Treatment regimen
Chemotherapy consisted of carboplatin (200 mg/m2 on treatment Day 1), oral etoposide (50 mg/body on treatment Days 2-22), and beta-interferon (300U/body on treatment Day1). This combination was administered and repeated every 4 weeks until tumor progression, provided that all hematological toxicities from the previous course had resolved to a Grade 2 or lower (NCI common toxicity criteria, version 3.0) and all other toxicities had recovered to either Grade 0 or 1. If enough recovery had not occurred, the subsequent course was delayed until these criteria were met; a delay of up to 2 weeks was allowed. No dose escalation was allowed. Dose reduction for toxicity was allowed. Glanisetron was given to all patients before administration of the chemotherapy regimen to prevent nausea and vomiting.
Evaluation of the treatment
The evaluation of the treatment was as follows: Complete Response (CR), complete disappearance of the enhanced area on MRI or CT; Partial Response (PR), more than 50 % reduction of the enhanced area on MRI or CT; Stable Disease (SD), less than 50 % reduction of the enhanced area on MRI or CT; Progressive Disease (PD), increasing enhanced area on MRI or CT. CR or PR was assessed as success of the treatment, and the ratio of success of the treatment was calculated. The survival period was examined from the date diagnosed as glioblastoma to December 31th in 2012.
Statistical analysis
Clinical biomarkers were analyzed by Kaplan-Meier survival analysis (using a log-rank test) and by multivariate analysis using a Cox proportional hazards regression model, estimating the adjusted hazard ratio (HR) with 95% confidence intervals (CI) for each variable relative to the risk of death or disease progression. Statistical analyses were performed using SPSS (IBM, New York, USA). In all cases, a P value of ≤.05 was regarded as significant.
RESULT Median follow-up period
The median follow-up period was 28.2 months (range 4 – 113.0 months).
Treatment responses
All patients could be assessed for a treatment response. There were 3 CRs (15%), 8 PRs (40%) to the CEI treatment. The overall response rate (CR+PR) was 55% (11 of 20 patients, 95% CI 43 67%). The median duration of disease stabilization was 24 weeks (range 6 – 60 weeks). The median duration of the overall response rate (CR+PR) was 28 weeks (range 9 -63 weeks). All responding patients were taking either a stable dose or no corticosteroids at the time of best response. The rate of 7 stable diseases was 40% (95% CI 28 -52%).
Disease progression
In all 20 enrolled patients, the median progression-free survival was 10.5 months (95% CI 7.1–13.9 months). Progression-free survival at 6 and 12 months after CEI treatment were 85%
No Diagnosis Age, Sex Location Surgery Response KPS PFS(m) OS(m)
1 Primary 55, M Pineal Biopsy CR 90 78 113+
2 Primary 78, M R parietal PRM SD 70 8 12
3 Primary 67, M R frontal STR PR 80 9 14
4 Primary 68, M L frontal GTR SD 80 11 15
5 Primary 50, F R frontal STR SD 60 12 19
6 Primary 57, M R frontal GTR PR 70 15 19
7 Secondary 41, M Pineal PRM SD 80 15 21
8 Primary 69, M R temporal GTR PR 70 6 11
9 Primary 61, M L temporal STR SD 70 7 14
10 Secondary 58, F R frontal, L parietal PRM SD 80 8 14
11 Secondary 61, F R temporal GTR SD 80 12 15
12 Primary 62, F L parietal STR PR 70 14 18
13 Primary 23, F L temporal GTR CR 90 10 16
14 Primary 59, M R temporal GTR SD 80 15 21
15 Primary 72, M R temporal STR PR 70 10 15
16 Primary 70, F L temporal STR PR 60 3 5
17 Primary 29, F L temporal GTR PR 80 9 15
18 Primary 59, M L temporo-occipital GTR CR 90 20 24
19 Secondary 22, M R temporo-occipital GTR PD 90 5 9
20 Secondary 37,M L frontal PRM PR 80 14 18
Table 1: Summary of patients’ undergone CEI therapy Case.

Central
Kanno et al. (2014)Email:
JSM Neurosurg Spine 2(3): 1027 (2014) 3/4
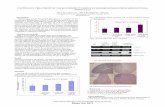
(95%CI 75-95%) and 45% (95% CI 32-58%), respectively (Figure 1).
Toxicity
Toxicities, which were graded based on the NCI common toxicity criteria (version 3.0), were recorded for all enrolled patients. The CEI regimen was generally well tolerated (Table 2). Grade 3 or 4 neutropenia occurred in 3 patients (15%), and Grade 3 or 4 thrombocytopenia occurred in 2 patients (10%). A lack of appetite or nausea occasionally persisted for 1 or 2 days, although the best antimetic agents were available to patients. Grade 3 or 4 hepatic and renal dysfunction were not observed in any patient. Nobody had encepaholopathy, pulmonary or auditory toxicity. Grade 1 or 2 alopecia was observed in 6 patients (30%).
Tolerability of regimen
The number of treatment cycles per patient ranged from 1-6. 3 patients received only 1 cycle, 4 received 2, 6 received 3, 4 received 4, 2 received 5, and 1 received 6.8
The median number of cycles per patient was 3.1. Treatment was discontinued in 6 patients because of disease progression, and 4 patients because of general condition.
Overall survival
The median survival time in the population studied, calculating from the start of the CEI chemotherapy, was 15.0 months (95% CI 6.8-23.2 months), and 85% (95% CI 72–98%) and 65% (95% CI 47–83%) of the patients were alive at 6 and 12 months after CEI treatment, respectively (Figure 1). On univariate analysis, there was a significant difference in the possibility of overall survival based on the extent of surgery (p = 0.001), but there was no difference on patient age (p = 0.45), or KPS score (p = 0.95).
DISCUSSION The first line chemotherapeutic agent in patients with
newly-diagnosed glioblastoma is temozolomide. However, once the tumor recurs or when the tumor expresses the O6-methylguanine DNA methlytransferase (MGMT), temozolomide has not enough effect for the tumor [13], and there is no established regimen. Temozolomide is alkylating agents, while carboplatin is a platinum compound and etoposide is an inhibitor of the enzyme topoisomerase II. Both carboplatin and etoposide have mechanism of action different alkylating agents like temozolomide. IFN-beta inhibits glioma cells through activation of caspase-7 and activation of DNase-gamma [14]. Since the mechanism of carboplatin, etpsoide, and interferon β against glioblastoma is different from that of temozolomide, the CEI regimen is expected to be effective in patients with glioblastoma after the no response to temolomide or nitorosourea-based regimens, and to be an alternative therapy for temozolomide-resistant glioblastoma.
In the chemotherapy using carboplatin and etoposide, it is useful that etopsoide is used with intravenous drip infusion in continuous 3-5 days. This chemotherapy is performed in the hospital. However, continuous several days of chemotherapy in the hospital impairs QOL of the glioma patient. Oral etoposide decreases the hospital stay and increases time of life at home and subsequently promotes QOL. In reported chemotherapies using oral etoposide, 25-50mg of etoposide /body of low dose showed higher efficacy and longer survival rather than over 100mg of relatively high dose. This reason might be due to characteristics plant alkaloid. In addition, carboplatin shows the highest level of chemosensitivity in glioblastoma and is easily used at the outpatient clinic, since it does not require hydration. Interferon β has not only direct inhibitory effect but also indirect immune effect against glioblastoma [15]. Although the number of case was small, this present study showed high efficacy rate and long progression free & overall survival. This result suggests that the CEI regimen of combination with carboplatin, etoposide, and interferon β is effective for gliblatoma.
REFERENCES1. Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ,
et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005; 352: 987-996.
2. Parney IF, Chang SM. Current chemotherapy for glioblastoma. Cancer J. 2003; 9: 149-156.
3. Ono A, Kanno H, Hayashi A, Nishimura S, Kyuma Y, Sato H, et al. Collagen gel matrix assay as an in vitro chemosensitivity test for malignant astrocytic tumors. Int J Clin Oncol. 2007; 12: 125-130.
Symptom Grade 1/2 No. of pts (%)
Grade 3/4 No. of pts (%)
anemia 4 (20.0) 1 (5.0)
thrombocytopenia 4 (20.0) 1 (5.0)
neutropenia 6 (30.0) 2 (10.0)
lymphocytopenia 3 (15.0) 0 (0)
nausea/vomiting 6 (30.0) 0 (0)transaminase
change 2 (10.0) 0 (0)
alopecia 6 (30.0) 0 (0)
constipation 3(15.0) 0 (0)
fatigue 10 (50.0) 1 (5.0)
diarrhea 3 (15.0) 0 (0)
anorexia 4 (20.0) 1 (5.2)
Table 2: Toxicities per patient following a CEI therapy.
Figure 1 Kaplan-Meier estimates of progression free survival (PFS) and overall survival (OS) in patients with GBMs undergone CEI therapy. Thick line, OS; Thin line, PFS.

Central
Kanno et al. (2014)Email:
JSM Neurosurg Spine 2(3): 1027 (2014) 4/4
Kanno H, Nakanowatari S (2014) Treatment for Glioblastoma with Carboplatin, Oral Etoposide and Interferon β (CEI): A Phase II Trial. JSM Neurosurg Spine 2(3): 1027.
Cite this article
4. Watanabe K, Kanaya H, Fujiyama Y, Kim P. Combination chemotherapy using carboplatin (JM-8) and etoposide (JET therapy) for recurrent malignant gliomas: a phase II study. Acta Neurochir (Wien). 2002; 144: 1265-1270.
5. Buckner JC, Brown LD, Cascino TL, Gerstner JB, Krook JE, Westberg MW, et al. Phase II evaluation of infusional etoposide and cisplatin in patients with recurrent astrocytoma. J Neurooncol. 1990; 9: 249-254.
6. Jeremic B, Grujicic D, Jevremovic S, Stanisavljevic B, Milojevic L, Djuric L, et al. Carboplatin and etoposide chemotherapy regimen for recurrent malignant glioma: a phase II study. J Clin Oncol. 1992; 10: 1074-1077.
7. Peterson K, Paleologos N, Forsyth P, Macdonald DR, Cairncross JG. Salvage chemotherapy for oligodendroglioma. J Neurosurg. 1996; 85: 597-601.
8. Franceschi E, Cavallo G, Scopece L, Paioli A, Pession A, Magrini E, et al.. Phase II trial of carboplatin and etoposide for patients with recurrent high-grade glioma. Br J Cancer. 2004; 91: 1038-1044.
9. Chamberlain MC. Recurrent brainstem gliomas treated with oral VP-16. J Neurooncol. 1993; 15: 133-139.
10. Chamberlain MC. Recurrent supratentorial malignant gliomas in
children. Long-term salvage therapy with oral etoposide. Arch Neurol. 1997; 54: 554-558.
11. Hainsworth JD. Extended-schedule oral etoposide in selected neoplasms and overview of administration and scheduling issues. Drugs. 1999; 58 Suppl 3: 51-56.
12. Brown PD, Maurer MJ, Rummans TA, Pollock BE, Ballman KV, Sloan JA, et al. A prospective study of quality of life in adults with newly diagnosed high-grade gliomas: the impact of the extent of resection on quality of life and survival. Neurosurgery. 2005; 57: 495-504.
13. Kim YS, Kim SH, Cho J, Kim JW, Chang JH, Kim DS, et al. MGMT gene promoter methylation as a potent prognostic factor in glioblastoma treated with temozolomide-based chemoradiotherapy: a single-institution study. Int J Radiat Oncol Biol Phys. 2012; 84: 661-667.
14. Saito R, Mizuno M, Hatano M, Kumabe T, Yoshimoto T, Yoshida J. Two different mechanisms of apoptosis resistance observed in interferon-beta induced apoptosis of human glioma cells. J Neurooncol. 2004; 67: 273-280.
15. Boiardi A, Silvani A, Milanesi I, Munari L, Broggi G, Botturi M. Local immunotherapy (beta-IFN) and systemic chemotherapy in primary glial tumors. Ital J Neurol Sci. 1991; 12: 163-168.