Synthesis of a Phenylphosphabenzene Ligand to … of a Phenylphosphabenzene Ligand to Improve a...
Click here to load reader
Transcript of Synthesis of a Phenylphosphabenzene Ligand to … of a Phenylphosphabenzene Ligand to Improve a...

Synthesis of a Phenylphosphabenzene Ligand to Improve a Cobalt Catalyst to Improve Dimerization for
Linear α-olefins
Jade Willey, 2017
Linear alpha olefins, or terminal alkenes, are used in the production of synthetic oils, detergents, and low-density
polyethylene. In 2012, North America accounted for 50% of world production. The current method used by industry to
produce linear alpha olefins is oligomerization. Oligomerization is a full-range process that produces linear alpha olefins
4-30+ carbons long. However, the most marketable linear alpha olefins contain 8-18 carbons. Dimerization is one method
being studied for the production of linear alpha olefins; e. g.1-hexene, is coupled with another 1-hexene, to produce 1-
dodecene. Dimerization could be a more efficient process, both economically and ecologically, than oligomerization.
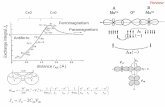
The Broene lab is modifying a cobalt catalyst coordinated to Cp*
(pentamethylcyclopentadienyl), trimethylphosphite, and ethene to be a more efficient
dimerization catalyst (shown top right). The cobalt catalyst has been shown to
produce a ratio of branched to linear alpha olefins of 4.5:1. Our hypothesis is that the
current ligand, the trimethylphosphite, which has a cone angle of 107° and freely
rotates in space, does not favor 2,1-insertion due to the sterics (shown bottom right).
Faculty Mentor: Rick Broene
The ligand we explored to facilitate dimerization is phenylphosphabenzene. The
phenylphosphabenzene has been shown to have a cone angle of 108, which is a
larger cone angle than the trimethylphosphite, however the cobalt can
backbond to the LUMO of the phenylphosphabenzene. This backbonding
hinders rotation, effectively eliminating the cone. By utilizing a ligand that will
be more planar, the complex will be sterically less hindered and will favor 2,1-
insertion, and therefore, more linear alpha olefins will be produced via
dimerization.
This summer, the immediate precursor to the phenylphosphabenzene was synthesized in five steps in 2.7% overall
yield. The phenyl(trimethylsilyl)ethynyl ketone was formed through palladium-catalyzed addition of trimethylsilyl (or
TMS) acetylene to benzoyl chloride in yields up to 100%. Grignard addition of ethynylmagnesium chloride to the
ketone formed the 3-hyrdoxy-3-phenyl-1-(trimethylsilyl)-1,4-pentadiyne in yields up to 84%. The alcohol was
methylated via phase transfer using 18-crown-6 and diemethylsulfate in yields up to 100%. Dibutyltin dihydride was
formed with LiAlH4 by reduction of dibutyltin dichloride in yields up to 47%. The hydride was used in the radical-
promoted (benzoyl peroxide) hydrostannylation of the 3-methoxy-3-phenyl-1,4-pentadiyne to form the 1,1-dibutyl-4-
methoxy-4-phenylstannacycle-2,5-diene (shown in HNMR below).
In the future, more experiments
involving the hydrostannylation
reaction exposed to oxygen will be
conducted to see if higher yields of
the stannacycle can be obtained.
After transmetalation of the tin for
the phosphorous atom and the
phenylphosphabenzene is
synthesized, future work will be to
coordinate the
phenylphosphabenzene to the
cobalt catalyst to see how efficient
the catalyst is at producing linear
alpha olefins.

Funded by: American Chemical Society, Petroleum Research Fund
References
1. Broene, R. D.; Brookhart, M.; Lamanna, W. M.; Volpe, A. F., Cobalt-catalyzed dimerization of α-olefins to give
linear α-olefin products. Journal of the American Chemical Society 2005, 127 (49), 17194-17195.
2. Tolman, C. A., “Steric Effects of Phosphorus Ligands in Organometallic Chemistry and Homogeneous
Catalysis.” Chem. Rev. 1977, 77 (3), 313-348.
3. Elschenbroich, C.; Nowotny, M.; Behrendt, A.; Harms, K.; Wocadlo, S.; Pebler, J., “Pentakis(η1-
phosphinine)iron: Synthesis, Structure, and Mode of Formation.” J. Am. Chem. Soc. 1994, 116 (14), 6217-6219.
4. Markl, G.; Kneidl, F., “Simple Synthesis of 4-Monosubstituted Arsa- and Phospha-benzenes.” Angew. Chem. Int.
Ed. Engl. 1973, 12 (11), 931-932.
5. Alpha Olefins (02/03-4), PERP Report, Nexant Chem Systems.
6. Alpha Olefin Market by Type and Applications – Global trends and Forecast To 2018. The Business Journals
Online. http://www.bizjournals.com/prnewswire/press_releases/2014/04/17/enUK201404178229 Accessed 18
July, 2015.
7. Tellmann, K. P.; Gibson, V. C.; White, A. J. P.; Williams, D. J., “Selective Dimerization/Oligomerization of α-
Olefins by Cobalt Bis(imino)pyridine Catalysts Stabilized by Trifluoromethyl Substituents: Group 9 Metal
Catalysts with Productivities Matching Those of Iron Systems.” Organometallics 2004, 24 (2), 280-286
8. Small, B. L.; Marcucci, A. J., “Iron Catalysts for the Head-to-Head Dimerization of α-Olefins and Mechanistic
Implications for the Production of Linear α-Olefins.” Organometallics 2001, 20 (26), 5738-5744.
9. Breit, B.;Winde, R.; Mackewitz,T.; Paciello, R.; Harms, K., “Phosphabenzenes as Monodentate p-Acceptor
Ligands for Rhodium-Catalyzed Hydroformylation.” Chem. Eur.J. 2001, 7 (14), 3106-3121.
10. Small, B. L., “Tridentate Cobalt Catalysts for Linear Dimerization and Isomerization of α-Olefins.”
Organometallics 2003, 22 (16), 3178-3183.
11. Lappin, G. R. Alpha Olefins Applications Handbook. Marcel Dekker: New York, 1989
12. Muller, C.; Vogt, D., “Phosphinines as ligands in homogeneous catalysis: recent developments, concepts and
perspectives”. Dalton Trans. 2007, 47, 5505-5523.
13. Oderinde, M.; Froese, R.; Organ, M., “2,2’-Azobis(2-methylpropionitrile)-Mediated Alkyne Hydrostannylation:
Reaction Mechanism”. Angew. Chem. Int. Ed. 2013, 52, 11334-11338.