static-content.springer.com10.1208... · Web viewSUPPLEMENTARY MATERIAL Layer-by-Layer Thin Films...
Transcript of static-content.springer.com10.1208... · Web viewSUPPLEMENTARY MATERIAL Layer-by-Layer Thin Films...

SUPPLEMENTARY MATERIAL
Layer-by-Layer Thin Films for Co-delivery of TGF-β siRNA and Epidermal Growth
Factor to Improve Excisional Wound Healing
Praveen Kumar Mandapalli, Suman Labala, Anup Jose, Shubhmita Bhatnagar, Renuka
Janupally, Dharmarajan Sriram, Venkata Vamsi Krishna Venuganti*
Department of Pharmacy, Birla Institute of Technology and Science (BITS) Pilani, Hyderabad
Campus, Hyderabad 500078, Telangana, India.
Physical and mechanical characterization of the LbL thin films
The sequential adsorption of chitosan and sodium alginate to form LbL thin films was monitored
using UV-visible spectroscopy and infrared spectroscopy. The results of this characterization are
presented as a separated article. The thickness of LbL thin films was measured using a digital
micrometer (Baker Gauges India Pvt. Ltd., Mumbai, India). The thickness was measured on
three different locations of a film and for at least three different films.
(a) Porosity. Porosity of the LbL thin film was evaluated by alcohol displacement
method. Briefly, small piece (1x1 cm2) of pre-weighed film was immersed in alcohol. After 24 h
the final weight of the film was recorded. The porosity was calculated using the Equation (1).S1
Porosity=(W 2−W 1)
ρV 1(1)
Where W1 and W2 indicate the weight of the polymer film before and after alcohol immersion,
respectively, and V1 is the volume before immersion, ρ is a constant for the alcohol density.
S1

(b) Degree of swelling. The swelling ratio of LbL thin film was determined after
suspension of film samples in phosphate buffered saline (PBS, pH 7.4) at room temperature for
24 h. The weight of samples was recorded before (dry weight, Wd) and after (wet weight, Ww)
immersion in PBS. Swelling ratio was calculated using the Equation (2).S1
Degree of swelling ( DS )=(Ww−Wd)Wd
×100 (2)
(c) Tensile strength. Tensile strength of the film was determined using tensile grips
(A/TG) attached to a texture analyzer (TA.XT, Plus, Stable Micro Systems, UK). The test film
was fixed to the upper tensile grip and load cell was tared to zero weight. The upper tensile grip
was moved to the preset distance of 25 mm and the test film was securely clamped to lower grip.
The tensile force was gradually applied on the test film till the film broke. The parameters
maintained were 1mm/s pretest speed, 1 mm/s test speed, in distance target mode.
Tensile strength =FmaxAfilm (3)
Where “Fmax” is the maximum force at breakage (N) and “Afilm” is the initial cross sectional area
of the sample (cm2). The percentage elongation was calculated using the Equation (4).
Elongation (%) = (L−L 0)
L 0×100 (4)
Where “L0” is the initial length of the specimen (cm) and “L” is the length at the moment of
rupture (cm). The elastic modulus was calculated using the Equation (5).
Elastic modulus = FlinAfilm
× 1ε (5)
Where “Flin” is the force at corresponding strain of the linear section (N), “Afilm” is the initial
cross sectional area of the film (cm2), and “ε” is the corresponding strain.
S2

(d) Burst strength. Film burst strength is the force required to break or rupture the
film. The burst strength of film was determined using film support rig fitted to a heavy duty
platform and the probe (3 mm cylindrical stainless steel) was connected to a 5 kg load cell. The
test was performed at a probe speed of 1 mm/s and an acquisition rate of 500 points/s. The force
applied increases until the film bursts and the peak force was recorded as film burst strength.
(e) Skin adhesion strength. The skin adhesion strength of the LbL thin film was
evaluated using C57BL/6 mouse skin as the model membrane. Mice were killed using excess
ether anesthesia and the dorsal abdominal skin was carefully harvested after clipping the hair.
The excised skin was affixed on a cylindrical perspex support and was secured with screws. The
LbL thin film (1 cm2) was adhered to a probe (1/2" cylindrical Delrin with part code P/0.5R)
using adhesive tape. The probe was lowered to make a contact with the skin tissue at a force of
0.2 N for a contact time of 30 s. It was then withdrawn at a rate of 0.1 mm/s to a distance of 5
mm. The data acquisition (500 points/s) and peak integration were performed using the XTRA
Dimension software package of the instrument. All the measurements were repeated at least
three times. The work of adhesion and the peak detachment force was used to evaluate the skin
adhesion strength of the films. The work of adhesion was calculated from the area under the
force-distance curve, and the peak detachment force was calculated as the maximum force
required for detaching the film from the skin.
Antibacterial activity of LbL thin films
The antibacterial activity of films was evaluated against Gram negative (Escherichia coli) and
Gram positive (Staphylococcus aureus) bacteria. The antibacterial activity of LbL thin films was
investigated by zone-of-inhibition method and spectrophotometric method. The pure cultures of
E. coli and S. aureus were sub-cultured in Luria-Bertani (LB) medium and Müeller-Hinton
S3

medium, respectively. These bacterial suspensions were incubated at 37 °C on a rotary shaker at
150 rpm. A cell count of 106 CFU (optical density of 0.2 at 600 nm wavelength) was achieved
after 12 h incubation.
For the zone-of-inhibition measurement, agar plate method was performed. The
components of the LB agar included tryptone, yeast extract, sodium chloride and 2% agar. The
LbL thin films were cut into discs of 0.8 cm diameter and placed over the LB agar seeded with
bacterial cultures. A standard antibiotic disc was used as a positive control. The standard
antibiotic disc contained chloramphenicol (C, 25 μg), streptomycin (S, 10 μg), and tetracycline
(TE, 25 μg). The plates were incubated at 37 °C for 24 h and examined for clear zone-of-
inhibition.
In spectrophotometric method, the films were cut into discs of 0.5 cm diameter and
placed in a 96 well plate. Tetracycline (10 µg/ml) was used as the positive control and blank
cells as negative control. The bacterial cell suspension (200 µl, optical density of 0.2 at 600 nm
wavelength) was added to the well containing polymer film. The growth kinetics of bacteria
were determined by measuring optical density at 600 nm wavelength using a micro-plate reader
(SpectraMax M4, Molecular Devices Inc., USA) at different time intervals including 0, 2, 4 and
6h.
The prolonged application of bandages for wound healing requires material that would
not be colonized by microorganisms. The antibacterial activity of polymer films was investigated
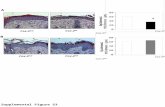
against E. coli and S. aureus as provided in the Supporting Information. Figure S1 shows the
zone-of-inhibition after incubation of LbL thin films compared with standard antibiotics. The
standards, chloramphenicol, streptomycin and tetracycline showed a zone-of-inhibition of 1.2 ±
0.2 cm, 0.9 ± 0.1 cm, 0.8 ± 0.2 cm against S. aureus and 1.0 ± 0.2 cm, 0.1 ± 0.02 cm, 1.1 ± 0.3
S4

cm against E. coli, respectively (Figure S1A and S1B). The LbL thin films showed a zone-of-
inhibition of 0.1 ± 0.01 cm and 0.1 ± 0.02 cm against both S. aureus and E.coli, respectively
(Figure S1C and S1D).
The bacterial growth kinetics in the presence of LbL thin film, chitosan film and sodium
alginate film was studied by measuring the optical density of bacterial cell suspension. Figure
S1E shows the percentage growth inhibition by polymer films after 6 h incubation for S. aureus
and E. coli. The E. coli growth in the presence of chitosan and LbL thin films was significantly
(p<0.05) less after 4 and 6 h incubation time in comparison with blank cells. The positive
control, tetracycline showed a growth inhibition of 82.8 ± 2.14% and 71.9 ± 3.27% against S.
aureus and E. coli, respectively. The chitosan and LbL thin film showed 36.1 ± 2.21%, 33.3 ±
1.32% and 10.9 ± 1.23%, 6.6 ± 1.23% growth inhibition against S. aureus, and E. coli,
respectively. It was found that LbL thin films and CS films are more effective against E. coli
compared with S. aureus. Sodium alginate did not show growth inhibition against both the
bacterial strains.
S5

Figure S1. Antibacterial activity of chitosan-alginate LbL thin film. Digital photographs of culture dishes to show zone-of-inhibition after treatment with standard antibiotics (A and B) and LbL thin films (C and D). Dotted circles represent the zone-of-inhibition from LbL thin films. E and F shows the growth curves of S. aureus and E. coli, respectively, in the presence of tetracycline, chitosan film (CS), sodium alginate film (SA) and LbL thin film up to 6 h. G. The percentage growth inhibition of S. aureus and E. coli after treatment with polymer films and
S6

tetracycline compared with bacterial growth without any treatment. All data points represent mean (n=3-4) ± standard deviation.
Reference
S1. P. T. Sudheesh Kumar, V. K. Lakshmanan, T. V. Anilkumar, C.
Ramya, P.
Reshmi, A. G.
Unnikrishnan, S. V.
Nair and R.
Jayakumar, ACS Appl. Mater. Interfaces, 2012, 4,
2618−2629.
S7

Table S1. Different treatment groups studied for application of TGF-β and EGF loaded LbL thin films.
Group Treatment Application
I No treatment None
II Blank LbL thin film Every day for 12 days
III Scrambled siRNA LbL thin film Every other day for 12 days
IV Commercial EGF gel Every day for 12 days
V EGF loaded LbL thin film Every day for 12 days
VI TGF-β siRNA loaded LbL thin film Every other day for 12 days
VII TGF-β siRNA and EGF loaded LbL thin film Every other day for 12 days
S8