Signal transduction mechanisms of HLA-DR-mediated interleukin-1β production in human monocytes
Transcript of Signal transduction mechanisms of HLA-DR-mediated interleukin-1β production in human monocytes

Signal Transduction Mechanisms of HLA-DR-Mediated Interleukin- 1/3 Production in Human Monocytes Role of Protein Kinase C and Tyrosine Kinase Activation
Tessa Palkama and Mikko Hurme
ABSTRACT: The signal transduction pathways leading" to the expression of IL-1/3 in human monocytes via HLA- DR stimulation were investigated. SEB, a staphylococcal enterotoxin that binds to HLA-DR molecules, induced IL-I~ expression in human monocytes. Protein synthesis inhibition by cycloheximide did not inhibit SEB-mediated IL-1/3 signal, indicating that protein synthesis is not re- quired for the MHC class-II-mediated IL-1/3 expression. The effect of PKC, PKA, and tyrosine kinase inhibitors on HLA-DR-mediated IL-1/3 mRNA expression was then determined. H7, a preferential PKC inhibitor, completely inhibited IL-1/3 signal induced by SEB. The role of PKC on HLA-DR-mediated IL-1/3 induction was further con- firmed by the ability of SEB to activate PKC on monocytes directly when measured with labeled phorbol ester
([3H]Pbt~)-binding capacity of whole cells. HA 1004, a preferential PKA inhibitor, and isobutyl-methyl-xanthine (IBMX), which inhibits the degradation of cAMP, had no effect on SEB-induced IL-1/3 signal, excluding the role of cAMP on HLA-DR-mediated IL-1/3 expression. Two tyrosine kinase inhibitors, genistein and dihydroxycinna- mate, both inhibited SEB-induced IL-1/3 mRNA in mono- cytes. SEB also induced enhanced tyrosine phosphoryla- tion of several proteins in human monocytes when determined with antiphosphotyrosine immunoblotting. Our results demonstrate that both PKC and protein tyro- sine kinases are involved in HLA-DR-induced IL-1/3 ex- pression in human monocytes. Human Immunology 36, 259-267 (1993)
ABBREVIATIONS AP-1 activating protein 1 BSA bovine serum albumin cAMP cyclic AMP [3H]Pbt2 [3H]phorbol-12,13~dibutyrate IBMX isobutyl-methyl-xanthine IL-1 interleukin 1 PBS phosphate-buffered saline PKA protein kinase A
PKC PMA pRGAPDH- 13
PTPase SEB TCR TRE
protein kinase C phorbol 12-myristate-13 acetate constant probe gtyceraldehyde
phosphate dehydrogenase phosphotyrosine phosphatase staphylococcal enterotoxin B T-cell receptor phorbol ester response element
I N T R O D U C T I O N
In addition to their role as restricting elements in antigen presentation, major histocompatibility complex (MHC)
From the Department of Bacteriology and Immunology, University of Helsinki, Helsinki, Finland.
Address reprint requests to Dr. T. Palkama, Department of Bacteriology and Immunology, University of HeIsinki, Haartmanink. 3, 00290 Hel- sinki, Finland.
Received (E) July 8, 1992; accepted November 23, /992.
class II molecules have been demonstrated to serve as signal-transducing receptors in many cell types. Both stimulatory and inhibitory effects of anti-class-II anti- bodies on B-cell proliferation were first reported [1, 2]. Ant i -MHC class II antibodies were shown to induce early biochemical signals, such as a rise in cyclic AMP (cAMP) accompanied with nuclear translocation of pro- tein kinase C (PKC) in murine B cells [3]. A mouse
Human Immunology 36, 259-267 (1993) 259 © American Society for Histocompatibility and Immunogenetics, 1993 0198-8859/93/$6.00

260 T. Palkama and M. Hurme
B-cell lymphoma expressing MHC class II molecules with truncated cytoplasmic domains was defective in anti-MHC class-Ii-induced PKC translocation [4] and this defect could be overcome by the addition of dibu- tyryl-cAMP [5]. Crosslinking of MHC class II antibodies induces the accumulation of inositol phosphates and an increase in intracellular calcium concentrations, and en- hances tyrosine phosphorylation in human B cells [6, 7]. Tyrosine phosphorylation and an increase in intracellular calcium have also been reported after anti-MHC class II stimulation in human T-cell clones and cell lines [8].
Monosyte-macrophages are the major source of in- terleukin 1 (IL-1) (reviewed by Dinarello [9]). IL-1 is an inflammatory cytokine produced after antigenic or infectious challenge. Two distinct molecular forms, IL- l~x and IL-1/3 have been characterized and their genes cloned and sequenced [10]. These molecules possess a variety of biologic activities in immune and inflammatory responses. IL-1 is the best-characterized costimulatory molecule in T-cell activation enhancing IL-2 secretion and inducing the expression of IL-2 receptors in T cells [11, 12]. Antigen-specific interaction of monocytes and T cells via the MHC class II molecule on the monocyte surface and the T-cell receptor (TCR)-CD3 complex on the T-cell surface has been suggested to mediate the signal required for IL-1 induction in monocytes at the initiation of antigen-specific immune response [13-15]. HLA-DR-binding antibodies and staphylococcal entero- toxins, which bind directly to the MHC class II mole- cules, also induce the production of IL-1 in human monocytes and myeloid cell lines [16-18].
The signal transduction pathways leading to IL-1 ex- pression have been partially characterized. PKC activa- tion has been reported to play an important role in the induction of IL-1 expression in various cell types [19, 20]. cAMP, acting presumably through activation of pro- tein kinase A (PKA), has also been reported to induce IL-1 expression in human monocytes [21] and murine peritoneal macrophages [22].
Tyrosine kinases are involved in the signal transduc- tion of many receptor systems and in the regulation of both normal and malignant cell growth (for a review, see Bolen [23]). Cellular proteins with tyrosine kinase activity may be divided into two major groups: those expressing extracellular ligand-binding domains and an intracellular tyrosine kinase domain (e.g., receptors for various polypeptide growth factors like platelet-derived growth factor receptor and macrophage colony-stimulat- ing factor [CSF] receptor) and those residing entirely intracellularly (e.g., members of the src, fes/fps, and abl gene families). The function of receptor tyrosine kinases is fairly well characterized, whereas the role ofintracellu- lar tyrosine kinases has remained elusive. Intracellular tyrosine kinases are differently expressed in distinct cell
lineages and in specific differentiational stages. Since high levels of some members of the src and fes family (e.g., hck, fyn, fes, and lyn) are expressed in terminally differentiated monocyte-macrophages [24-26], intra- cellular tyrosine kinases have been suggested also to mediate activation signals in these cells. Recently bacte- rial lipopolysaccharide was shown to induce tyrosine phosphorylation in monocytes [27] and granulo- cyte-macrophage CSF, which activates protein tyrosine phosphorylation, induces I L l production in human mononuclear cells [28].
We have reported that HLA-DR antibodies induce IL-1 production in human monocytes [ 17]. In this work, the signal transduction mechanisms of HLA-DR-medi- ated IL-1/3 expression were characterized. The role of PKC and cAMP, which both are activated via MHC class II molecules in lymphocytes, was investigated. Since ty- rosine kinase inhibitors were recently shown to inhibit MHC class-II-mediated IL-1 production in the myeloid cell line THP-1 [29], we also determined whether pro- tein tyrosine kinases are involved in IL-1/3 expression via HLA-DR molecules in human monocytes.
MATERIALS A N D M E T H O D S
Reagents. Staphylococcal enterotoxin B (SEB) was pur- chased from Toxin Technology (Sarasota, FL, USA). Phorbo112-myristate-13-acetate (PMA), isobutyl-meth- yl-xanthine (IBMX), and sodium orthovanadate were purchased from Sigma Chemical Company (St. Louis, MO, USA). PKC inhibitor H7 (1-[5-isoquinolone-sul- fonyl]-2-methylpiperazine dihydrochloride) and PKA inhibitor HA1004 (N-[2-guanidinoethyl]-5-isoquinol- inesulfonamide hydrochloride) were purchased from Seikagaku Kogyo (Tokyo, Japan). Genistein and methyl 2,5-dihydroxycinnamate were purchased from Gibco Research Products Life Technologies (Gaithersburg, MD, USA).
Cell cultures. Leukocyte-enriched buffy coats were ob- tained from the Finnish Red Cross Blood Transfusion Service (Helsinki, Finland). Mononuclear cells were iso- lated from the buffy coats by Ficoll-Isopaque (Phar- macia, Uppsala, Sweden) centrifugation. The cells were suspended at 10V/ml in RPMI-1640 medium (Flow labo- ratories, Irvine, Scotland), supplemented with Hepes, 10% human AB serum (Finnish Red Cross Blood Trans- fusion Service), 10 mM L-glutamine, and antibiotics (complete medium). In RNA analysis, mononuclear cells from each buffy coat were directly divided into the ex- perimental groups indicated in 90-mm Petri dishes. After 1 hour, nonadherent cells were removed by wash- ing the dishes twice with warm RPMI-1640 supple- mented with Hepes and 10 ml of complete medium and

IL-1/3 Expression Via HLA-DR in Human Monocytes 261
the indicated stimulators were added. To avoid interindi- vidual variations between buffy coats in RNA analysis, each experimental group consisted of cells derived from six buffy coats. At times indicated, monocytes were har- vested by adding cold phosphate-buffered saline (PBS) (Ca 2+, Mg 2+ free; Orion Diagnostica, Espoo, Finland) to the cells and scraping with a rubber policeman.
RNA isolation and analysis. Adherent monocytes were harvested and total cellular RNA was isolated by guani- dium isothiocyanate lysis and CsC1 centrifugation [30, 31]. The RNA isolated was quantitated spectrophoto- metrically and 30-/zg samples were size-fractionated in 1.2 % agarose-formaldehyde gels, transferred to a nylon membrane (Pall, Glen Cove, NY, USA), dried, and baked at 80°C. The IL-1/3 cDNA probe (HU-IL-1/3, pcDSRa) used was provided by Dr. Kari Varkila (DNAX Research Institute, Palo Alto, CA, USA). The RNA levels on the nylon membranes were also quantitated by using a constant probe, glyceraldehyde phosphate dehydrogenase (pRGAPDH-13). The latter was a gift from Dr. Kari Alitalo (Department of Pathology, Uni- versity of Helsinki). The IL-1/3 cDNA insert was purified from Barn H 1-digested fragments of the HU-IL-1/3 plas- mid, and the GAPDH insert was purified from Pst 1-digested fragments from pRGAPDH-13 plasmid. In- serts were labeled with 32p by using a random-primed DNA-labeling kit purchased from Boehringer Mann- heim (Mannheim, Germany). Prehybridizations and hy- bridizations were performed in a solution containing 50% formamide, 5 x Denhardt's solution, 5 x SSPE, and 0.5% SDS. Filters were washed in l x SSC plus 0.1% SDS twice for 30 minutes at room temperature and then at 60°C for 30 minutes. Subsequently, the filters were exposed to Kodak AR X-Omat films at -70°C with intensifying screens.
Preparation of cell lysates and antiphosphotyrosine immu- noblotting. Mononuclear cells were prepared as de- scribed above and cells from each huffy coat were di- rectly divided into the experimental groups indicated in 50-mm Petri dishes. Nonadherent cells were removed after 1 hour by washing the dishes twice with warm RPMI-1640 supplemented with Hepes. Cells were se- rum deprived by incubating them overnight in 5 ml RPMI-1640 medium supplemented with 10 mM L-glutamine and antibiotics. After that, the indicated stimulators were added. To inhibit phosphotyrosine phosphatase (PTPase) activity, 0.1 mM sodium ortho- vanadate and 2 mM H202 were added for 20 minutes after stimulation as described previously [32]. Cells were then washed in situ with 37°C PBS and lysed in 125/zl boiling lysis buffer (2.5% SDS, 0.125) M Tris-HCl pH 6.8, and 1 mM sodium orthovanadate). Lysates were
then boiled for 3 minutes, sonicated for 15 seconds, and centrifuged for 10 minutes (14,000 rpm) at 4°C. Protein concentration of the lysates was determined with BCA Protein Assay (Pierce, Rockford, IL, USA) and 25 /.tg protein from each experimental group was electropho- resed in 12% SDS-polyacrylamide minigels under re- ducing conditions (Bio-Rad Laboratories, Richmond, CA, USA). Separated proteins were transferred to Hybond-C nitrocellulose membranes (Amersham, UK). Nonspecific binding was blocked by incubating the fil- ters overnight at 3% (BSA)-PBS in room temperature. Filters were then incubated for 4 hours with polyclonal rabbit antiphosphotyrosine antibody (Upstate Biotech- nology, Lake Placid, NY, USA), washed three times with 0.1% Tween-PBS, and incubated for 1 hour with peroxidase-conjugated goat-anti-rabbit immuno- globulin G (Jackson Immunoresearch Laboratories, West Grove, PA, USA). After washing, immunoreactive proteins were detected with enhanced chemilumines- cence (ECL) (Amersham) according to the manufactur- er's instructions and the filters were exposed to Kodak AR X-Omat films.
PKC assay. Activation of PKC was determined by esti- mation of phorbol-ester-binding capacity of whole cells as described [33]. Briefly, mononuclear cells were di- rectly adhered to confluency on microtiter plates and nonadherent cells were removed as described above and 0.2 ml complete media was added to each well. Adherent cells were treated at 37°C with the indicated stimulators for various time periods. Cells were then washed with PBS and divided into two groups, and incubated for 10 minutes at 37°C in PBS containing 4 mg/ml BSA and 40 nM [~H]phorbol- 12,13-dibutyrate ([3H]Pbt2; Radio- chemical Center, Amersham) with or without 3 mM of unlabeled competitor PMA. Cell-nonassociated [3H]PBt 2 was washed away with PBS-BSA. Specific binding was estimated by subtracting nonspecific binding in the presence of 250-fold excess of unlabeled PMA. The data are shown as the mean fold of increase +- standard error of the mean of specific binding in stimu- lated cells when compared with the specific binding in unstimulated cells. Each group was tested in triplicate. The experiment was repeated three times with similar results.
RESULTS
SEB, a ligand binding to the HLA-DR molecule, induces IL- 1/3 expression in human monocytes, which is not dependent on protein synthesis. Human monocytes were cultured with 1/zg/ml SEB, which is known to bind with high affinity to the HLA-DR region of HLA class II molecule [34] and has been reported to stimulate THP-1 cells, which

262 T. Palkama and M. Hurme
m ILl ¢/J
_~ + "O =D 3< X O I l l Z "1" IE ~ ~ O
IL -1B
GAPDH
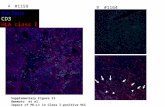
FIGURE 1 SEB induces IL-1/3 expression in human mono- cytes that is not inhibited by cycloheximide. Human monocytes were cultured with media or with 10 /~g/ml cycloheximide (CHX), a protein synthesis inhibitor. After 1 hour, cells were left unstimulated or stimulated with 1/zg/ml SEB for an addi- tional 3 hours. Total cellular RNA was isolated and analyzed for IL-1/3 expression as described in Materials and Methods. Each experimental group contains adherent mononuclear cells from six blood donors, and the experiment was repeated three times with similar results.
mediated IL-1/3 production was tested. Cells were prein- cubated for 30 minutes with the protein kinase inhibitors prior to stimulation with 1/~g/ml SEB and analyzed for their IL-1/3 mRNA expression. A total of 25 /~M H7, a concentration that effectively inhibits phorbol-ester- induced IL-1/3 production [37], completely inhibited IL- l[3 expression induced by SEB, while the same concen- tration of H A 1004 had no effect on SEB-mediated IL- l/3 expression (Fig. 2). SEB was stimulated also in the presence of IBMX, a cAMP phosphodiesterase inhibi- tor, which inhibits the degradation of cAMP and has been reported to upregulate cAMP-dependent IL-1 sig- nal [21, 37]; 0.5 mM IBMX had no effect on SEB- mediated IL-1/3 expression (results not shown), also speaking against a cAMP-mediated signal in HLA-DR- mediated IL-1/3 induction.
SEB activates PKC on human monocytes when measured with phorbol-dibutyrate-binding activity. Since the pro- tein kinase inhibitors used may exert other effects in addition to their specific kinase-inhibiting capacities, we also tested the ability of SEB to activate PKC on human monocytes directly. PKC activation was determined by phorbol-ester-binding capacity of whole cells as de- scribed in Materials and Methods. This assay determines
express HLA class II molecules [18]. SEB was unable to stimulate IL-1 production in the monocytic cell line THP-1 with a low expression of MHC class II molecules (results not shown), which seems to exclude non-MHC class-II-mediated effects of SEB in monocytic cells. In line with our own published results and the work of others [16-18] , ligand binding to HLA class II mole- cules induced the expression of IL-113 mRNA in human monocytes. SEB-mediated IL-1/3 expression was in- duced rapidly, the maximum IL-1/3 mRNA level was reached after 3 hours (Fig. 1) and IL-1/3 expression de- clined in 24 hours (results not shown).
SEB-mediated IL-1/3 induction did not require pro- tein synthesis, as it was not inhibited by the protein synthesis inhibitor cycloheximide (CHX). In fact, CHX, which alone slightly induced IL-1/3 mRNA, as reported previously [35, 36], had a superinducing effect on SEB- mediated IL-1/3 expression.
SEB-mediated IL-1 [3 expression is inhibited by PKC inhibi- tors, while PKA inhibitors and agents elevating intracellular cAMP have no effect on IL-1/3 induction. PKA and cAMP have both been reported to regulate IL-1/3 gene expres- sion [ 19, 21, 22]. To characterize the signal transduction mechanisms leading to HLA-DR-mediated IL-1/3 ex- pression, the effect of H7, a preferential PKC inhibitor, and H A 1004, a preferential PKA inhibitor, on SEB-
FIGURE 2 SEB-mediated IL-1/3 expression is inhibited by PKC inhibitor H7, while PKA inhibitor HA 1004 has no effect on IL-1B induction. Monocytes were preincubated for 30 minutes with 25/~M H7 or HA 1004 prior to stimulation with 1 ~g/ml SEB for 3 hours. Cells were collected and ana- lyzed for their IL-1/3 mRNA expression as described in Materi- als and Methods. Each experimental group contains adherent mononuclear cells from six blood donors, and the experiment was repeated three times with similar results.
O O
• 'r "r + ÷
J
O i l l iU Ill E o~ ~a
IL-18
GAPDH

IL-lfl Expression Via HLA-DR in Human Monocytes 263
O~ .m "0 ¢:
=t ,.D "0
E
0 J~ I . 0
J¢:
31 2"
4 8 1 '2 1 '6 2'0 2'4 28
Time (min)
FIGURE 3 SEB activates PKC on human monocytes when measured with phorbol-dibutyrate-binding activity. Activation of PKC was determined by estimation ofphorbol-ester-binding capacity of whole cells. Mononuclear cells were directly ad- hered to microtiter plates, and nonadherent cells were re- moved after 1 hour. Adherent cells were left untreated or treated as triplicates at 37°C with 1/zg/ml SEB for the time periods indicated. Cells were then washed with PBS, divided into two groups, and incubated for 10 minutes at 37°C in PBS containing 4 mg/ml BSA and 40 nM [3H]phorbol-12,13- dibutyrate ([3H]Pbt2) with or without 3 mM of unlabeled com- petitor PMA. Cell-nonassociated [3H]PBt2 was washed away with PBS-BSA. Specific binding was estimated by subtracting nonspecific binding in the presence of 250-fold excess of unla- beled PMA. The data are shown as the mean fold of increase + standard error of the mean of specific binding in stimulated cells when compared with the specific binding in unstimulated cells. Each sample was tested in triplicate and the experiment was repeated three times with similar results.
PKC activation by binding of labeled phorbol dibutyrate to activated PKC [38]. SEB induced [3H]Pbt2-binding activity at 20 minutes on human monocytes, and the binding activity declined to baseline during the 30-mi- nute assay time (Fig. 3).
The role of tyrosine kinase inhibitors on HLA-DR-mediated IL-l f l expression. Tyrosine phosphorylation has been shown to occur in MHC class-II-mediated signal trans-
duction in lymphocytes [6-8] and myeloid cell lines [29]. We thus wanted to explore the role of tyrosine kinases in HLA-DR-mediated IL-lfl induction in human monocytes. Two different inhibitors of tyrosine phos- phorylation, methyl 2,5-dihydroxycinnamate and gen- istein were tested on their ability to modify SEB-medi- ated IL-1/3 induction. Monocytes were preincubated for 15 minutes with the indicated concentrations of methyl 2,5-dihydroxycinnamate or for 30 minutes with 30/zg/ ml genistein. After that, cells were stimulated with 1/~g/ ml SEB for 3 hours and analyzed for their IL-lfl mRNA expression. Both methyl 2,5-dihydroxycinnamate and genistein had an inhibitory effect on SEB-mediated IL- lfl expression, indicating that tyrosine kinases mediate HLA-DR-induced IL-lfl signal (Fig. 4).
SEB induces tyrosine phosphorylation in human mono- cytes. To characterize HLA-DR-mediated tyrosine ki- nase activation further, the effect of SEB on protein tyrosine phosphorylation in human monocytes was de- termined using antiphosphotyrosine immunoblotting (Fig. 5). Human adherent monocytes were first stimu- lated with 1/zg/ml SEB for the indicated time periods. After that, cells were treated for an additional 20 minutes with 0.1 mM sodium orthovanadate and 2 mM H20 z to inhibit tyrosine phosphatases and amplify tyrosine phosphorylation signal as described previously [32]. SEB induced protein tyrosine phosphorylation in 10 minutes, the most prominent bands corresponding to proteins of approximately 56 and 73 kD. Reproducible, but weaker, bands were detected at the molecular range of 130-105 kD and at 31 and 39 kD. The tyrosine- phosphorylating effect of SEB was sustained, as en- hanced tyrosine phosphorylation was still detectable after 30 minutes of SEB stimulation.
DISCUSSION
The signal transduction pathways leading to IL-1/3 ex- pression in human monocytes via MHC class II mole- cules were investigated. SEB, a staphylococcal entero- toxin, which has been shown to bind with high affinity to HLA-DR molecules [34] and induce IL-lfl expression in human monocytes and the monocytic cell line THP-1 [18], was used as the MHC class II ligand.
H7, a preferential PKC inhibitor, completely inhib- ited SEB-induced IL-lfl signal, indicating that activation of PKC is critical in MHC class-II-mediated IL-lfl pro- duction. The role of PKC in MHC class-II-mediated monocyte activation was further confirmed by the ability of SEB to activate PKC directly when measured with labeled phorbol-ester-binding capacity on whole cells. This assay determines PKC activity through binding of labeled phorbol dibutyrate to activated PKC [38]. PKC

264 T. Palkama and M. Hurme
A
• ... .,..
=L 0 Q
• o
E • R gll m
C C C
+ + +
"o m m m, ¢1 ¢D @ IJJ I~I i~J ~J u.l
B
:d.
0
w
Q
+
m U,I ¢/)
I L -1B
G A P D H
FIGURE 4 Tyrosine kinase inhibitors genistein and 2,5-dihydroxycinnamate in- hibit HLA-DR-mediated IL-lfl expression. Monocytes were preincubated for 15 mi- nutes with the indicated concentrations of methyl 2,5-dihydroxycinnamate (A) or for 30 minutes with 30 /~g/ml genistein (B). After that, cells were stimulated with 1/zm/ ml SEB for 3 hours and analyzed for their IL-lfl mRNA expression. Each experimen- tal group consists of cells from six blood donors, and the experiment was repeated three times with similar results.
signal is mediated by different transcription factors, which bind to specific cis elements on PKC-activated genes [39]. The best characterized of these PKC-depen- dent pathways is mediated by the fos/jun (activating protein 1; AP-1) transcription factor complex, which binds to phorbol ester response (TRE) elements in the genes following PKC activation [40]. It has recently been demonstrated that IL- 1/3 gene contains a functional TRE element [41]. However, transcriptional regulation of especially c-fos expression is considered to be crucial at the initiation of AP-l-mediated responses [39]. It is thus interesting that, unlike with phorbol ester stimula- tion, inhibition of translation by cyclohexmide has a su- perinducing effect on SEB-mediated IL-1/3 expression. This suggests that alternative PKC-dependent transcrip- tion factors, independent of translation (e.g., NF-KB [42]), would function in HLA-DR-mediated IL-1/3 ex- pression. The IL-1/3 gene has a binding site for NF-t<B [43], but its function has not been demonstrated.
An increase in intracellular cAMP and subsequent nuclear translocation of PKC were first demonstrated in murine lymphoid cells after MHC class-II-mediated stimulation [3]. We have reported previously that sus- tained elevation of intracellular cAMP upregulates IL-1 expression in human monocytes, while transiently ele- vated cAMP is not a sufficient signal for IL-1 expression [21].
To determine whether cAMP had a role in MHC class-II-mediated IL-1/3 expression, the effect of HA
1004, a preferential PKA inhibitor, and IBMX, an inhib- itor of cAMP phosphodiesterase that is well documented to upregulate cAMP-dependent IL-1 signal in human monocytes [21, 37], was investigated. HA 1004 and IBMX had no effect on the mRNA levels induced by SEB, indicating that cAMP is not involved in MHC class- II-mediated IL-1/3 induction. Also, the rapid kinetics of SEB induced IL-1/3 expression, which declines in 24 hours, speaks against the involvement of cAMP in MHC class-II-mediated IL-1/3 induction, since the kinetics of cAMP-induced IL-1 expression in human monocytes is very slow, with maximal mRNA expression at 24 hours [21].
Data on protein tyrosine phosphorylation via MHC class II in different cell types exist [6-8]. Recently IL- 1/3 expression induced via MHC class II in the myeloid cell line THP-1 was shown to be inhibited by tyrosine kinase inhibitors [29]. Since members of the intracellu- lar protein tyrosine kinases (e.g., src family) are differen- tially expressed during myeloid differentiation [24-26], we investigated whether tyrosine kinases are also in- volved in MHC class-II-mediated IL-1/3 induction in differentiated monocytes. The effect of two tyrosine kinase inhibitors, which act via different mechanisms, genistein and methyl 2,5-dihydroxycinnamate, was tested on SEB-induced IL-1/3 expression. Both genistein and methyl 2,5-dihydroxycinnamate had an inhibitory effect on SEBdnduced IL-1/3 expression in human monocytes. Low concentrations (2/.tg/ml) of cinnamate,

IL-1/~ Expression Via HLA-DR in Human Monocytes 265
E E o o
"o m m ¢~ uJ UJ :E (n (n
- 97 ~*"'°~ -66
-45
, -31
-21
-14
FIGURE 5 The effect of SEB on tyrosine phosphorylation in human monocytes. Human adherent monocytes were stimu- lated for 10 or 30 minutes with 1/zg/ml SEB. Cells were then treated for an additional 20 minutes with 0.1 mM sodium orthovanadate and 2 mM H202. After that, cells were lysed, separated in 12% SDS-polyacrylamide gel, transferred to ni- trocellulose, and immunoblotted with antiphosphotyrosine and peroxidase-conjugated second antibody. Immunoreactive proteins were detected with enhanced chemiluminescence and autoradiography.
which have previously been reported not to inhibit tyro- sine kinases in vitro [44], enhanced IL-1/3 expression. This indicates that cinnamate, like genistein [45], may also have other, non-tyrosine-kinase-inhibiting effects.
The protein tyrosine phosphorylation pattern in- duced by SEB in antiphosphotyrosine immunoblots was also characterized, and SEB was found to induce sus- tained tyrosine phosphorylation of several proteins. Pro- tein phosphorylation on tyrosine was elevated in 10 minutes and remained still detectable at 30 minutes. The activity of tyrosine kinases is intimately controlled by PTPases, and PTPase activity increases during myeloid differentiation [46]. This was also evident in our experi- ments, since reliable detection of proteins phosphory- lated on tyrosine required the presence of vanadate and H202 , which have a synergistic effect on the inhibition of PTPases [32].
Our data clearly indicate that PKC and tyrosine ki- nases are both involved in the MHC class-II-mediated IL-1 signal. In T-lymphocytes, tyrosine kinase and PKC pathways are both activated in TCR-mediated signal transduction. Tyrosine phosphorylation is required for phospholipase-C-mediated inositol phospholipid hydro- lysis via TCR-CD3. Since TCR-CD3 complex lacks intrinsic tyrosine kinase activity, nonreceptor tyrosine kinases associated with the complex seem to be responsi-
ble for the tyrosine phosphorylation. Members of the src family; p59fyn associated with the TCR-CD3 com- plex and p561ck associated with the CD4 or CD8 mole- cules are the most likely candidates to mediate the tyro- sine phosphorylation signal (reviewed by Mustelin and Altman [47]). A similar activation cascade has been sug- gested to be involved in MHC class-II-mediated signals in lymphocytes. An increase in intracellular calcium by MHC class II antibodies is abrogated by crosslinking class II molecules with CD45, a receptor PTPase [8]. Thus, tyrosine kinases in these lymphocyte activation models work upstream of PKC activation. However, data indicating that PKC regulated tyrosine phosphory- lation also exist. PMA, a direct PKC activator, induces protein tyrosine phosphorylation [48], and tyrosine phosphorylation of lymphoid microtubule-associated protein-2 kinase depends on PKC [49]. We are currently investigating the role of PKC and tyrosine kinases in the transcriptional activation of MHC class-II-induced IL-1/3 expression. Further work will reveal whether PKC and tyrosine kinase signal transduction pathways are cou- pled or function independently of each other in MHC class-II-mediated IL-1/3 production in human mono- cytes.
The characterization of MHC class-II-mediated IL-1 signal transduction pathways in peripheral blood mono- cytes is of in vivo significance, because these cells are considered the major antigen-presenting cells at the initi- ation of the immune response. T cells are required to mediate the IL-l-inducing signal during antigen-specific interaction of monocytes and T cells, since protein anti- gens alone do not induce IL-1 production [13]. It is of interest that this restriction is broken with staphylococ- cal enterotoxins (e.g., SEB) that belong to the microbial superantigens. Microbial superantigens can bind class II molecules on the MHC complex and specific TCR V/3 gene products on T cells, making them powerful T-cell activators [34, 50]. The linkage of several autoimmune diseases, such as rheumathoid arthritis and juvenile-on- set diabetes, to specific MHC class II alleles has sug- gested the involvement of class-II-restricted T cells in them. Recently, data on the involvement of a superanti- gen were reported on rheumathoid arthritis [51]. Due to the inflammatory effects of IL-1, the possibility of MHC class-II-mediated IL-1 production in the patho- genesis of these diseases is of considerable clinical in- terest.
ACKNOWLEDGMENTS
The authors thank Mr. Sami Starast for technical assistance. This work was supported by grants from the Finnish Cultural Foundation, the Paulo Foundation, the Finnish Cancer Society, the Finnish Medical Foundation Duodecim, and Leiras Medical Corporation.

266 T. Palkama and M. Hurme
REFERENCES
1. Forsgren S, Pobor G, Coutinho A, Pierres M: The role of I-A/E molecules in B lymphocyte activation. J Immunol 133:2104, 1984.
2. Cambier JC, Lehmann KR: Ia-mediated signal transduc- tion leads to proliferation of primed B lymphocytes. J Exp Med 170:877, 1989.
3. Cambier JC, Newell MK, Justement LB, McGuire JC, Leach KL, Chen ZZ: Ia binding ligands and cAMP stimu- late nuclear translocation of PKC in B lymphocytes. Na- ture 327:629, 1987.
4. Nabavi N, Ghogawala Z, Myer A, Griffith IJ, Wade WF, Chen ZZ, McKean DJ, Glimcher LH: Antigen presenta- tion abrogated in cells expressing truncated Ia molecules. J Immunol 142:1444,1989.
5. St-Pierre Y, Nabavi N, Ghogawala Z, Glimcher LH, Watts TH: A functional role for signal transduction via the cyto- plasmic domains of MHC class II proteins. J Immunol 143:808, 1989.
6. Lane PJL, McConnell FM, Schieven GL, Clark EA, Led- better JA: The role of class II molecules in human B cell activation: association with phosphatidyl inositol turnover, protein tyrosine phosphorylation, and proliferation. J Im- munol 144:3684, 1990.
7. Mooney NA, Grillot-Courvalin C, Hivroz C, Ju L-Y, Charron D: Early biochemical events after MHC class II- mediated signaling on human B lymphocytes. J Immunol 145:2070, 1990.
8. Odum N, Martin PJ, Schieven GL, Norris NA, Grosmaire LS, Hansen JA, Ledbetter JA: Signal transduction by HLA-DR is mediated by tyrosine kinase(s) and regulated by CD45 in activated T cells. Hum Immuno132:85, 1991.
9. Dinarello CA: Interleukin-1 and interleukin-1 antago- nism. Blood 77:1627, 1991.
10. March CJ, Mosley B, Larsen A, Cerretti DP, Braedt G, Price V, Gillis S, Henney CS, Kronheim SR, Grabstein K, Conlon PJ, Hopp TP, Cosman D: Cloning, sequence and expression of two distinct human interleukin-1 com- plementary DNAs. Nature 315:641, 1985.
11. KayeJ, Gillis S, Mizel SB, Shevach EM, Malek TR, Dinar- ello CA, Lachman LB, Janeway CAJ: Growth of a cloned helper T cell line induced by a monoclonal antibody spe- cific for the antigen receptor: interleukin 1 is required for the expression of receptors for interleukin 2. J Immunol 133:1339, 1984.
12. LowenthalJW, CerrotiniJ-C, MacDonald HR: Interleukin 1-dependent induction of both interleukin 2 secretion and interleukin 2 receptor expression by thymoma cells. J Immunol 137:1226, 1986.
13. Weaver CT, Unanue ER: T cell induction of membrane IL 1 on macrophages. J Immunol 137:3868, 1986.
14. Wasik MA, Donnelly RP, Belier DI: Lymphokine-inde- pendent induction of macrophage membrane IL-1 by au- toreactive T cells recognizing either class I or class II. J Immunol 141:3456, 1988.
15. Landis RC, Friedman ML, Fisher RI, Ellis TM: Induction of human monocyte IL-1 mRNA and secretion during anti-CD3 mitogenesis requires two distinct T cell-derived signals. J Immunol 146:128, 1991.
16. Palacios R: Monoclonal antibodies against human Ia anti- gens stimulate monocytes to secrete interleukin 1. Proc Natl Acad Sci USA 82:6652, 1985.
17. PalkamaT, Sihvola M, Hurme M: Induction of Interleukin lce (IL-lce) and IL-1/3 mRNA expression and cellular IL-1 production by anti-HLA-DR antibodies in human mono- cytes. Scand J Immunol 29:609, 1989.
18. Trede NS, Geha RS, Chatila T: Transcriptional activation of IL-1/3 and tumor necrosis factor-c~ genes by MHC class II ligands. J Immunol 146:2310, 1991.
19. Kovacs EJ, Radzioch D, Young HA, Varesio L: Differen- tial inhibition of IL-1 and TNF-ce mRNA expression by agents which block second messenger pathways in murine macrophages. J Immunol 141:3101, 1988.
20. Strulovici B, Daniel-Issakani S, Oto E, Nestor J, Chan H, Tsou A-P: Activation of distinct protein kinase C iso- enzymes by phorbol esters: correlation with induction of interleukin 1/3 gene expression. Biochemistry 28:3569, 1989.
21. Serkkola E, Hurme M, Palkama T: Prolonged elevation of intracellular cyclic AMP activates interleukin-1 produc- tion in human peripheral blood monocytes. ScandJ Immu- nol 35:203, 1992.
22. Ohmori Y, Strassrnan G, Hamilton TA: cAMP differen- tially regulates expression of mRNA encoding IL-lc~ and IL-1/3 in murine peritoneal macrophages. J Immunol 145:3333, 1990.
23. Bolen JB: Signal transduction by the SRC family of tyro- sine protein kinases in hemopoietic cells. Cell Growth Differ 2:409, 1991.
24. Ziegler SF, Wilson CB, Perlmutter RM: Augmented ex- pression ofa myeloid-specific protein tyrosine kinase gene (hck) after macrophage activation. J Exp Med 168:1801, 1988.
25. Smithgall TE,Yu G, Glazer RI: Identification of the differ- entiation-associated p93 tyrosine protein kinase of HL-60 leukemia cells as the product of the human c-fes locus and its expression in myelomonocytic cells. J Biol Chem 263:15050, 1988.
26. Katagiri K, Katagiri T, Koyama Y, Morikawa M, Yama- moto T, Yoshida T: Expression ofsrc family genes during monocytic differentiation of HL-60 cells. J Immunol 146:701, 1991.
27. Weinstein SL, Gold MR, DeFranco AL: Bacterial lipopo- lysaccharide stimulates protein tyrosine phosphorylation in macrophages. Proc Natl Acad Sci USA 88:4148, 1991.
28. Sisson SD, Dinarello CA: Production of interleukin-lex, interleukin-1/3 and tumor necrosis factor by human mono- nuclear cells stimulated with granulocyte-macrophage colony-stimulating factor. Blood 72:1368, 1988.

IL-1/3 Expression Via HLA-DR in Human Monocytes 267
29. Scholl PR, Trede N, ChatilaTA, Geha RS: Role of protein tyrosine phosphorylation in monokine induction by the staphylococcal superantigen toxic shock syndrome toxin-1. J Immunol 148:2237, 1992.
30. Glisin V, Crkvenjakov R, Byus C: Ribonucleic acid iso- lated by cesium chloride centrifugation. Biochemistry 13:2633, 1974.
31. Chirgwin JM, Przybula AE, MacDonald RJ, Rutter WJ: Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry 18:5294, 1979.
32. Heffetz D, Bushkin I, Dror R, Zick Y: The insulinomi- metic agents H202 and vanadate stimulate protein tyrosine phosphorylation in intact cells. J Biol Chem 265:2896, 1990.
33. Schiitze S, Nottrott S, Pfizenmaier K, Kr6nke M: Tumor necrosis factor signal transduction: cell-type-specific acti- vation and translocation of protein kinase C. J Immunol 144:2604, 1990.
34. Fraser JD: High-affinity binding of staphylococcal entero- toxins A and B to HLA-DR. Nature 339:221, 1989.
35. Turner M, Chantry D, Buchan G, Barrett K, Feldmann M: Regulation of expression of human IL-lc~ and IL-1/~ genes. J Immunol 143:3556, 1989.
36. Schindler R, Clark BD, Dinarello CA: Dissociation be- tween interlukin-1/3 and protein synthesis in human pe- ripheral blood mononuclear cells. J Immuno1265:10232, 1990.
37. Palkama T: Induction of interleukin-1 production by li- gands binding to the scavenger receptor in human mono- cytes and the THP-1 cell line. Immunology 74:432, 1991.
38. Dougherty RW, Niedel JE: Cytosolic calcium regulates phorbol diester binding affinity in intact phagocytes. J Biol Chem 261:4097, 1986.
39. Angel P, Karin M: The role of Jun, Fos and the AP-1 complex in cell-proliferation and transformation. Biochim Biophys Acta 1072:129, 1991.
40. Angel P, Imagawa M, Chiu R, Stein B, Imbra RJ, Rahms- dorf HJ, Jonat C, Herrlich P, Karin M: Phorbol ester- inducible genes contain a common cis element recognized by a TPA-modulated trans-acting factor. Cell 49:729, 1987.
41. Bensi G, Mora M, Raugei G, Buonamassa DT, Rossini M, Melli M: An inducible enhancer controls the expression of the human interleukin 1/3 gene. Cell Growth Differ 1:491, 1990.
42. Sen R, Baltimore D: Inducibility of K immunoglobulin enhancer-binding protein NF-KB by a posttranslational mechanism. Cell 47:921, 1986.
43. Clark BD, Collins KL, Gandy MS, Webb AC, Auron PE: Genomic sequence for human prointerleukin 1 beta: possible evolution from a reverse transcribed prointerleu- kin 1 alpha gene. Nucleic Acids Res 14:7897, 1986.
44. Umezawa K, Hori T, Tajima H, Imoto M, Isshiki K, Takeuchi T: Inhibition of epidermal growth factor-in- duced DNA synthesis by tyrosine kinase inhibitors. FEBS Lett 260:198, 1990.
45. Makishima M, Honma Y, Hozumi M, Sampi K, Hattori M, Umezawa K, Motoyoshi K: Effects of inhibitors of protein tyrosine kinase activity and/or phosphatidylinosi- tol turnover on differentiation of some human myelomo- nocytic leukemia ceils. Leuk Res 15:701, 1991.
46. Frank DA, Sartorelli AC: Regulation of protein phospho- tyrosine content by changes in tyrosine kinase and protein phosphotyrosine phosphatase activities during induced granulocytic and monocytic differentiation of HL-60 leu- kemia cells. Biochem Biophys Res Commun 140:440, 1986.
47. Mustelin T, Altman A: Tyrosine phosphorylation in T<ell activation. Scand J Immunol 34:259, 1991.
48. Gilmore T, Martin GS: Phorbol ester and diacylglycerol induce protein phosphorylation at tyrosine. Nature 306:487, 1983.
49. Nel AE, Hanekom C, Hultin L: Protein kinase C plays a role in the induction of tyrosine phosphorylation of lym- hold microtubule-associated protein-2 kinase. J Immunol 147:1933, 1991.
50. Choi YW, Herman A, DiGiusto D, Wade T, Marrack P, Kappler J: Residues of the variable region of the T-cell- receptor beta-chain that interact with S. aureus toxin super- antigens. Nature 346:474, 1990.
51. Paliard X, West SG, Lafferty JA, Clements JR, Kappler JW, Marrack P, Kotzin BL: Evidence for the effects of a superantigen in rheumatoid arthritis. Science 253:325, 1991.