PneumaCult -Ex Plus, a Novel Defined and Feeder-Free ...€¦ · 2) Time (Sec) Bronchial...
Transcript of PneumaCult -Ex Plus, a Novel Defined and Feeder-Free ...€¦ · 2) Time (Sec) Bronchial...

0
5
10
15
20
25
30
35
0 1000 2000 3000 4000 5000 6000Is
c (μ
A/c
m2 )
Time (Sec)
Bronchial epithelial growth medium
PneumaCult™-Ex
PneumaCult™-Ex Plus
0
100
200
300
400
500
600
700
800
900
P3 P4 P5 P6 P7 P8
TEER
Avg
(Ω x
cm
²) Bronchial epithelial growth medium
PneumaCult™-Ex
PneumaCult™-Ex Plus
P5
PneumaCult™-Ex Plus, a Novel Defined and Feeder-Free Medium, Supports the Improved Expansion of Primary Human Airway Epithelial Cells and Maintenance of their Mucociliary Differentiation Potential in Later PassagesJuan Hou1, Tyler Brown1, Arisa Yoshikawa1, Michael J. Riedel1, John Stingl1, Terry E. Thomas1, Allen C. Eaves1,2, and Sharon A. Louis1
1 STEMCELL Technologies Inc., Vancouver, Canada 2 Terry Fox Laboratory, BC Cancer Agency, Vancouver, B.C., Canada
Introduction
Materials and Methods
Traditional feeder-free and Bovine Pituitary Extract (BPE)-containing media formulations for the expansion of primary human bronchial epithelial cells (HBECs) typically support the maintenance of their mucociliary differentiation potential for a limited number of passages in vitro. A novel culture system comprising an inactivated mouse embryonic fibroblast feeder layer and a specialized medium has been recently reported to improve the expansion of HBECs while maintaining their differentiation potential, even after extended passaging1,2. However this feeder-dependent method is cumbersome and undefined, thus limiting its utility. We have developed a novel feeder- and BPE-free culture medium, PneumaCult™-Ex Plus, that promotes extended passaging of HBECs without the loss of their differentiation potential at later passages. PneumaCult™-Ex Plus allows for the rapid expansion of HBECs, while maintaining their ability to form a pseudostratified mucociliary epithelium at air-liquid interface (ALI) for more passages, compared to other commercial HBEC expansion media.
FIGURE 1: PneumaCult™ culture system
Commercially available primary normal human airway epithelial cells such as Lonza’s HBECs [Passage (P)1; Catalog #CC-2540s] were thawed and seeded directly into T-25 cm2 culture flasks containing either PneumaCult™-Ex Plus, PneumaCult™-Ex or commercial bronchial epithelial growth medium at a density of 3.5 x 103 cells/cm2. Culture media were fully replenished every other day and cultures were passaged once cells reached approximately 80% confluence. At each passage, the cells were enzymatically dissociated using Trypsin-EDTA (0.05%) and then re-plated at a density of 5 x 103 cells/cm2 in the diffrerent medium. Fold expansion was measured over 8 passages and the differentiation potential was assessed at each passage.
FOR RESEARCH USE ONLY. NOT INTENDED FOR HUMAN OR ANIMAL DIAGNOSTIC OR THERAPEUTIC USES. STEMCELL TECHNOLOGIES INC.’S QUALITY MANAGEMENT SYSTEM IS CERTIFIED TO ISO 13485 MEDICAL DEVICE STANDARDS. Scientists Helping Scientists™ | WWW.STEMCELL.COM
TOLL-FREE PHONE 1 800 667 0322 • PHONE 1 604 877 0713 • [email protected] • [email protected]
FOR GLOBAL CONTACT DETAILS VISIT OUR WEBSITE
PneumaCult™-Ex Plus is a serum- and BPE- free medium that supports higher expansion of HBECs compared to a commercial
expansion media
HBECs expanded in PneumaCult™-Ex Plus maintain better mucociliary differentiation potentials
PneumaCult™-Ex, together with PneumaCult™-ALI, creates an optimized BPE-free culture system for expanding and differentiating
HBECs into a pseudostratified mucociliary epithelium, resembling the human airway both morphologically and
electrophysiologically
Conclusions
Results
Commercially available, cryopreserved, passage 1 (P1) HBECs were seeded into PneumaCult™-Ex Plus, PneumaCult™-Ex or commercial bronchial epithelial growth medium. Cells cultured in PneumaCult™-Ex Plus have a significantly higher proliferation rate over 9 passages compared to those maintained in either PneumaCult™-Ex or commercial bronchial epithelial growth medium (n=6).
FIGURE 3: Representative morphology of HBECs cultured in PneumaCult™-Ex Plus, PneumaCult™-Ex, orBronchial epithelial growth medium
Representative live culture images for P4 HBECs cultured in PneumaCult™-Ex plus or control media (PneumaCult™-Ex or commercial bronchial epithelial growth medium). Compared to the cells cultured in PneumaCult™-Ex (B) and commercial bronchial epithelial growth medium (C), HBECs cultured in PneumaCult™-Ex Plus (A) are smaller and more tightly packed. All images were taken using 10X objective.
FIGURE 4: HBECs cultured in PneumaCult™-Ex Plus maintain widespread expression of the basal cell markers CD49f and CD271
Immunocytochemistry detection of basal cell markers CD49f (A, B, and C) and CD271 (D, E,
and F) for P4 HBECs cultured in PneumaCult™-Ex Plus (A and D), PneumaCult™-Ex (B and E), and commercial bronchial epithelial growth medium (C
and F). All images were taken using 10X objective. Nuclei are counterstained with DAPI.
FIGURE 5: HBECs cultured in PneumaCult™-Ex Plus maintain a larger population of CD271+CD49f+ basal cells
P4 HBECs cultured in PneumaCult™-Ex Plus (A), PneumaCult™-Ex (B), and commercial bronchial epithelial growth medium (C) were characterized by flow cytometry for expression of the basal cell markers CD49f and CD271. HBECs cultured in PneumaCult™-Ex Plus (A) have a higher percentage of cells co-expressing CD49f and CD271 compared to those cultured in PneumaCult™-Ex (B) and commercial bronchial epithelial growth medium (C).
FIGURE 6: HBECs cultured in PneumaCult™-Ex Plus differentiate into a pseudostratified mucociliary epithelium at later passages in PneumaCult™-ALI
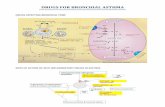
FIGURE 7: Electrophysiological characterization of differentiated HBECs previously expanded in PneumaCult™-Ex Plus, PneumaCult™-Ex, and commercial bronchial epithelial growth medium
P4 HBECs were seeded and passaged in either PneumaCult™-Ex Plus, PneumaCult™-Ex, or commercial bronchial epithelial growth medium, followed by ALI differentiation at each passage (P5 - 8) with PneumaCult™-ALI Medium. The ALI cultures at 28 days post air-lift were fixed and stained with antibodies for cilia marker acetylated-tubulin (red) and the goblet cell marker MUC5AC (green). The nuclei are counterstained with DAPI (blue). All images were taken using 20X objective.
(A) Transepithelial electrical resistance (TEER) for ALI cultures at 28 days post air-lift using HBECs expanded in either PneumaCult™-Ex Plus, PneumaCult™-Ex, or commercial bronchial epithelial growth medium. (B) Representative characterization of the ion channel activities for the ALI cultures at 28 days post air-lift using HBECs expanded in PneumaCult™-Ex Plus, PneumaCult™-Ex, or commercial bronchial epithelial grown medium. Amiloride: Epithelial Sodium Channel (ENaC) inhibitor. IBMX and Forskolin: Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) activators. Genistein: CFTR potentiator. CFTRinh-172: CFTR inhibitor. UTP: Calcium-activated Chloride channels (CaCCs) activator. ISC: Short Circuit Current. All ALI differentiation cultures were performed using PneumaCult™-ALI Medium.
Air-Liquid Interface Culture Procedure
PseudostratifiedEpithelium
Primary HBECs
Submerged Culture Procedure
Expansion Phase(Submerged Culture
in Inserts)
Maintenance Phase(ALI Culture in Inserts)
2 - 4 days 21+ days3 - 5 days per passage
Apical Chamber
Basal Chamber 0.4 μm-pore membrane
Expansion Culture(Submerged Culture
in Flasks/Wells)
PneumaCult™-Ex Plus PneumaCult™-Ex Plus PneumaCult™-ALI
A B C
PneumaCult™-Ex Plus Bronchial epithelial growth mediumPneumaCult™-Ex
PneumaCult™-Ex PlusBronchial epithelial
growth mediumPneumaCult™-Ex
CD49f
CD271
C01 Ex+.fcsSinglets
FITC-A
AP
C-A
102
103
104
105
106
100
101
102
103
104
105
106
107
0.05%0.46%
86.64%12.85%
PneumaCult™-Ex Plus
CD49
f-APC
CD271-FITC
B06 Ex 1.0.fcsSinglets
FITC-A
AP
C-A
102
103
104
105
106
100
101
102
103
104
105
106
107
0.14%1.23%
9.91%88.71%
PneumaCult™-Ex
CD49
f-APC
CD271-FITC
B05 BEGM.fcsSinglets
FITC-A
AP
C-A
102
103
104
105
106
100
101
102
103
104
105
106
107
0.18%2.74%
5.88%91.17%
CD49
f-APC
CD271-FITC
Bronchial epithelial growth mediumA B C
A B C
D E F
Passage
A B
A B C D
E F G
H I
PneumaCult™-Ex Plus
PneumaCult™-Ex
Bronchial epithelialgrowth medium
P5 P6 P7 P8
References1. Liu X, et al. (2012) Am J Pathol 180(2): 599-6072. Suprynowicz F, et al. (2012) PNAS (109): 20035-20040
0.00
5.00
10.00
15.00
20.00
25.00
30.00
0 1 2 3 4 5 6 7 8 9
Popu
latio
n D
oubl
ings
Passage Number
Bronchial epithelial growth mediumPneumaCult™-ExPneumaCult™-Ex Plus
FIGURE 2: HBECs cultured in PneumaCult™-Ex Plus showed more rapid expansion compared to those cultured in PneumaCult™-Ex and commercial bronchial epithelial growth medium
Amiloride
IBMX+
Forskolin
Genistein
CFTRinh-172
UTP
MUC5AC = Goblet cells
AC-tubulin = Ciliated cellsDAPI = Nuclei
I SC (µ
A/c
m2 )