Novel brominated flame retardants and dechloranes in three fish species from the St. Lawrence River,...
Transcript of Novel brominated flame retardants and dechloranes in three fish species from the St. Lawrence River,...
Science of the Total Environment 479–480 (2014) 48–56
Contents lists available at ScienceDirect
Science of the Total Environment
j ourna l homepage: www.e lsev ie r .com/ locate /sc i totenv
Novel brominated flame retardants and dechloranes in three fish speciesfrom the St. Lawrence River, Canada
Magali Houde a,⁎, David Berryman b, Yves de Lafontaine a, Jonathan Verreault c
a Environment Canada, Centre Saint-Laurent, 105 McGill Street, Montreal, QC H2Y 2E7, Canadab Direction du suivi de l'état de l'environnement, Ministère du Développement durable, de l'Environnement, de la Faune et des Parcs, 675, boul. René-Lévesque Est, 7e étage, Québec, QC G1R 5V7, Canadac Centre de recherche en toxicologie de l'environnement (TOXEN), Département des sciences biologiques, Université du Québec à Montréal, C.P. 8888, Succursale Centre-ville, Montreal,QC H3C 3P8, Canada
H I G H L I G H T S
• 38 PBDE congeners and 16 emerging flame retardants were analyzed in St. Lawrence River fish.• Eight non-PBDE flame retardants could be detected in the liver of northern pike and muskellunge.• For the first time, Dec 604 Compound B was quantified in fish.• Sum of PBDE concentrations was up to 24 μg/g l.w. in apex predator.
⁎ Corresponding author. Tel.: +1 514 496 6774; fax: +E-mail addresses: [email protected] (M. Houde),
[email protected] (D. Berryman), yvesd(Y. de Lafontaine), [email protected] (J. Verrea
0048-9697/$ – see front matter. Crown Copyright © 2014http://dx.doi.org/10.1016/j.scitotenv.2014.01.105
a b s t r a c t
a r t i c l e i n f oArticle history:Received 26 November 2013Received in revised form 27 January 2014Accepted 27 January 2014Available online 15 February 2014
Keywords:Emerging flame retardantsDechloranesPolybrominated diphenyl ethersSt. Lawrence RiverFish
Restrictions in the utilization of polybrominated diphenyl ether (PBDE)mixtures have led to the increased usageof alternative flame retardant additives in a wide range of commercial applications. The present study examinedthe occurrence of established and emerging flame retardants (FRs) in fish from a densely-populated urbanizedsector of the St. Lawrence River (Montreal, Quebec, Canada). Thirty-eight PBDE congeners and sixteen emergingFRs were determined in fish belonging to three predatory species (yellow perch, northern pike, andmuskellunge).The∑PBDE in fishwere up to 24,115 ng/g lipid weight (l.w.) in the apex predatormuskellunge. Twelve emergingFRs including bis(2-ethylhexyl)-tetrabromophthalate (BEHTBP), pentabromoethylbenzene (PBEB), DechloranePlus (anti and syn), dechloranes (Dec) 602, Dec 604, Dec 604 Compound B (Dec 604 CB), and Chlordene Plus(CP)were detected (N0.01 ng/g l.w.) in the liver ofmuskellunge and northern pike but not in yellow perch homog-enates. This is the first report of Dec 604 CB in any fish species. The bioavailability of these FRs in human-impactedaquatic ecosystems warrants further environmental assessment and toxicity testing.
Crown Copyright © 2014 Published by Elsevier B.V. All rights reserved.
1. Introduction
Fire safety standards have led to the use of a series of halogenatedflame retardants (FRs) in a wide range of commercial and industrialproducts. Polybrominated diphenyl ethers (PBDEs) have been usedsince the late 1970s in such applications. These compounds wereshown to bioaccumulate and biomagnify in wildlife (Shaw and Kannan,2009; de Wit et al., 2010; Ma et al., 2012), to be toxic (Darnerud, 2003)and exhibit increasing trends in concentrations over time in some species(Crosse et al., 2012; Dietz et al., 2012; Hoguet et al., 2013). The recentbans of penta- and octa-BDE formulations, and the anticipated phase-out of deca-BDE by the end of 2013, have led to the increased production
1 514 496 7398.
Published by Elsevier B.V. All rights
and use of alternative halogenated and non-halogenated FRs for whichinformation on the environmental fate, toxicokinetics and toxicity islimited.
Emerging brominated FR additives are included in several tech-nical mixtures. Chemical components such as 2-ethylhexyl 2,3,4,5-tetrabromobenzoate (EHTBB), bis(2-ethylhexyl)-tetrabromophthalate(BEHTBP), 1,2-bis(2,4,6,-tribromophenoxy)ethane (BTBPE) and deca-bromodiphenyl ethane (DBDPE) are used in Firemaster formulations(Chemtura Corporation). These FRs have recently been reported in var-ious environmental matrices including house dust (Stapleton et al.,2008), sediment (Klosterhaus et al., 2012), fish (Munschy et al., 2011),birds (Gentes et al., 2012; Guerra et al., 2012), mammals (Klosterhauset al., 2012), and even human serum (Cequier et al., 2013). In additionto environmental detection, biomagnification potential has been re-ported for DBDPE (Law et al., 2006), which is considered an alternativeto deca-BDE for plastic and textile applications because of similar phys-icochemical properties (Covaci et al., 2011). The biomagnification of
reserved.
49M. Houde et al. / Science of the Total Environment 479–480 (2014) 48–56
other emerging FRs (e.g. BTBPE, EHTBB, and pentabromoethylbenzene(PBEB)) has also been observed in aquatic food webs (Tomy et al.,2007a; de Wit et al., 2010; Wu et al., 2010a).
Dechlorane Plus (DP)was introduced on themarket as a replacementfor Dechlorane (i.e., Mirex) which was formerly used as insecticide andflame retardant. Although DP has been used for at least 40 years in plas-ticizer, polyvinylchloride, neoprene, and electrical coating, it has onlybeen reported since 2006 in abiotic and biotic samples, includinghuman milk (Siddique et al., 2012 and reviews by Sverko et al., 2011and Feo et al., 2012). Bioaccumulation and biomagnification have beenreported for DP (Tomy et al., 2007b, 2008; Wu et al., 2010b) as well aslong-range atmospheric transport, based on air samples collected frompole-to-pole (Möller et al., 2010). DP is considered to be a high produc-tion volume (HPV) chemical under the U.S. EPA HPV Challenge Program(http://www.epa.gov/chemrtk/) and is on Canada's Domestic SubstancesList (http://www.ec.gc.ca/substances/nsb/search/eng/cp_search_e.cfm).It was included in the list of high priority chemicals with persistenceand bioaccumulation potential by Howard and Muir (2010) in additionto other FRs, such as BEHTBP, octabromo-1,3,3-trimethyl-1-phenylindan(OBIND), and DBDPE.
Additional dechloranes, including Dec 602 and Dec 604, were usedas substitutes of mirex and also patented as FRs (Feo et al., 2012) andhave most likely been used since the 1960s. These chemicals wereonly recently reported in sediment and muscle tissues of lake troutand whitefish from the Laurentian Great Lakes (Shen et al., 2010,2011a,b). Dec 602 and 603 were found in sediment, oyster, and eelpoutfromEastern andNorthern China (Jia et al., 2011;Wang et al., 2012; Sunet al., 2013) as well as in liver of franciscana dolphins from Brazil (de laTorre et al., 2012). Dec 602 was also detected in air, soil and sedimentnear a DP production facility in China (Wang et al., 2010) and degrada-tion products were identified in blubber of Canadian Arctic belugawhales and lake trout and whitefish from the Great Lakes (Shen et al.,2012). Chlordene Plus (CP), for which very little information is current-ly available on the production, is being used as an additive in plastic andhas also been reported in sediment (Shen et al., 2011b) and dolphin (dela Torre et al., 2012). Dec 602 andDec 604 are listed on the EnvironmentCanada's Nondomestic Substances List (http://www.ec.gc.ca/lcpecepa/default.asp?lang=En&n=8BD37498-1) and the European ChemicalSubstances Information System suggesting actual commercial usage(ESIS). Other related compounds, such as Dec 604 Component B(Dec 604 CB), have as yet not been reported in any environmentalsamples. No information could be found on the production and useof Dec 604 CB.
The St. Lawrence River is the second most important waterway inNorth America connecting the Great Lakes to the Atlantic Ocean. Urbaneffluents in the vicinity of Montreal island (Quebec), the most populatedarea of this Canadian province, have been reported as significant sourcesof PBDEs to the river ecosystem (Pelletier and Rondeau, 2013). Highdetection frequencies of novel FRs (BEHTBP: 89%, anti-DP: 100%, andsyn-DP: 93%)were recently reported in liver of ring-billed gulls collecteddownstream of Montreal in addition to high concentrations of PBDEsincluding BDE-209 (Gentes et al., 2012). A Canada-wide study onherring gulls also reported the highest PBDE concentrations in eggscollected from an island just downstream of Montreal (Chen et al.,2012). No data are currently available for emerging FRs in fish fromthis area.
Given the lack of information on non-PBDE FRs in aquatic biota, aproject was elaborated to investigate the occurrence of a compre-hensive suite of PBDE congeners and selected emerging PBDE-alternative FRs in fish from the St. Lawrence River in the greaterMontreal area. Brominated and chlorinated FRs were determined intissues of yellow perch (Perca flavescens), northern pike (Esox lucius)and muskellunge (Esox muskellunge). Fish species were selectedbased on their wide distribution in the study area, their apex trophicposition, and their recreational and economic importance to thisregion.
2. Material and methods
2.1. Fish sampling
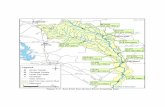
Yellow perch (n = 29), northern pike (n = 11), and muskellunge(n = 10) were collected between 2008 and 2012 at various sites inthe St. Lawrence River around Montreal (Fig. 1). Sampling siteswere selected upstream and downstream of Montreal as well as intwo tributaries (Du Nord River and L'Assomption River) in order toevaluate possible areas of concern. Several wastewater treatment efflu-ent outfalls, hypothesized to be important sources of non-PBDE andPBDE contamination, were located near study sites in addition tothe major Montreal's waste water treatment plant (WWTP) dis-charge located at the eastern end of the island (Fig. 1). Lake desDeux-Montagnes is located at the mouth of the Ottawa River,which is a major tributary, and Lake St-Louis is a fluvial lake receivingwaters from the Great Lakes in the southern end and from the OttawaRiver on its north side. The Mille Iles River connects Lake desDeux-Montagnes to the St. Lawrence River. Capture methods consistedof standard angling equipment, beach seine, and electricfishing. Samplescollected were used in several research projects and tissue processingdiffered between species, thus liver (northern pike and muskellunge)and whole fish homogenate (yellow perch) were available for the envi-ronmental assessment and kept at −20 °C until analysis. The age offish was determined by counting the number of growth annuli usingcleithra (pike) and opercula (perch) while age of muskellunge was es-timated based on length. Fish size, weight, and age are reported inTable 1. Fish body condition was estimated using the Fulton's conditionfactor = (weight (g) / (total length (cm)3) × 1000.
2.2. Chemical analysis
Fish liver and whole homogenate samples were analyzed for38 PBDE congeners and 16 non-PBDE FRs including pentabromotoluene(PBT), pentabromoethylbenzene (PBEB), bis(2-ethylhexyl)-tetra-bromophthalate (BEHTBP), hexabromobenzene (HBB), deca-bromodiphenyl ethane (DBDPE), 2,2′,4,4,5,5′-hexabromobiphenyl(BB-153), octabromo-1,3,3-trimethyl-1-phenylindan (OBIND), 1,2-bis(2,4,6,-tribromophenoxy)ethane (BTBPE), 2-ethylhexyl 2,3,4,5-tetrabromobenzoate (EHTBB), Dechloranes (Dec) 602, 603, 604,Dechlorane 604 Compound B (Dec 604 CB), Chlordene Plus (CP) andboth isomers of Dechlorane Plus (syn-DP and anti-DP) (Table 1 of theSupplementary information, SI). Briefly, sample aliquots (0.75–1.0 g)were ground with diatomaceous earth, spiked with 100 μL of a 200 ppbinternal standard solution (BDE-30, BDE-156, 13C-BDE-209, 13C-syn-DP and 13C-anti-DP), and extracted with dichloromethane:n-hexane(50:50) using a pressurized liquid extraction system (PLE) (FluidManagement Systems, Watertown, MA). Lipid content was determinedgravimetrically. Sample clean-up was achieved using a PBDE-free silicaacid-basic-neutral (ABN) column followed by a PBDE-free neutral alumi-na column (Fluid Management Systems) eluted with dichloromethane:n-hexane (50:50). Identification and quantification of target analyteswere performed using a gas chromatograph (GC) coupled to a singlequadrupole mass spectrometer (MS) (Agilent Technologies 5975C Series,Palo Alto, CA) operating in the electron capture negative ionizationmode(GC/MS-ECNI) based on methods described by Gentes et al. (2012).The analytical column (15 m × 0.25 mm × 0.10 μm) was a fused-silicaDB-5 HT capillary column (J & W Scientific, Brockville, ON, Canada).
Quality control and assurance procedures included proceduralmethod blanks, repeated analyses of fish samples and standard refer-ence material (SRM 1947; Lake Michigan fish tissue) for each batch often samples. Blank correction was performed for PBDE and non-PBDEFRs and concentrations can be found in SI Table 1. Mean (±SE) internalstandard recoveries from fish liver and homogenate, blanks and SRM1947 samples were as follows: BDE-30 (88.7 ± 1.83%), BDE-156(94.6 ± 3.45%), 13C-BDE-209 (58.4 ± 4.0%), 13C-syn-DP (91.2 ± 3.32%)
Fig. 1. Fish sampling sites in the St. Lawrence River and two tributaries in the vicinity of Montreal (Quebec, Canada). The river runs from south-west to north-east. Sampling sites (●),effluent outfalls (★), and the Montreal's WWTP effluent outfall (★) are indicated.
50 M. Houde et al. / Science of the Total Environment 479–480 (2014) 48–56
and 13C-anti-DP (91.1 ± 3.41%). Concentrations of PBDEs and emergingFRs were quantified using an internal standard approach, and thus allanalyte concentrations were inherently recovery-corrected. PBDE con-centrations determined in SRM 1947 showed less than 26% deviationfrom certified values. Method limits of detection (MLODs; definedas signal to noise ratio (S/N) = 3) and method limits of quantifica-tion (MLOQs; minimum amount of analyte producing a peak withS/N= 10) were based on replicate analyses (n = 8) of matrix samplesspiked at a concentration of 3–5 times the estimated detection limit.
Table 1Sampling location and fish characteristics (range) for specimens analyzed.
Fish species Sampling location Sampling year
Yellow perch Boucherville islands 2012Ilet Vert 2012Beauregard island 2012du Nord River 2012Mille Iles River 2012L'Assomption River 2012
Northern pike Lake des Deux 2011MontagnesIlet Vert 2011Mille-Iles River 2012
Muskellunge Lake St-Louis 2009Varennes 2008–09Lake des Deux 2010MontagnesOttawa River 2010–11du Nord River 2012
na: not available.
2.3. Data analysis
The arithmetic mean concentration of individual compound wascalculated when at least 50% of the samples from each species had con-centrations above the compound-specific MLOQ. When this criterionwas met, samples with concentrations below the MLOD were assigneda random value between zero and the compound-specific MLOD,while samples with concentrations below the MLOQ were assigned arandom value between the MLOD and the MLOQ. Concentrations were
N Length (mm) Weight (g) Age (years)
6 146–178 31–63 2–36 155–175 40–71 26 152–188 40–73 2–46 187–227 77–158 na3 159–231 43–161 na2 260–270 213–226 na5 567–789 1116–3023 3–7
4 570–807 1329–5196 2–42 487–589 1040–1209 –
2 1309–1334 10,527–15,575 N72 1010–1100 7424–10,848 N73 960–1342 6128–16,800 N7
2 1219–1239 11,433–12,872 N71 507 746 na
Table2
Meanconc
entrations
(ng/glip
idweigh
t)±
stan
dard
errorof
flam
eretardan
tsde
tected
inye
llow
perchwho
leho
mog
enates
andliv
erof
northe
rnpike
andmuske
llung
efrom
theSt.Law
renc
eRive
ran
dtributaries(200
8–20
12).Ra
nges
aregive
nwhe
nco
ncen
trations
inb50
%of
samples
werebe
low
MLO
Qs.Aco
ncen
trationisindicatedwhe
nthech
emical
was
detected
inon
lyon
efish
.
Chem
ical
Metho
dlim
itof
quan
tification
(MLO
Q)
Yello
wpe
rch(n
=29
)Northernpike
(n=
11)
Mus
kellu
nge(n
=10
)
%sampleNMLO
QMean±
S.E.
(orrang
e)%sampleNMLO
QMean±
S.E.
(orrang
e)%sampleNMLO
QMea
n±
S.E.
(orrang
e)
Lipidco
nten
t(%
)3.6±
0.56
7.5±
1.2
15±
4.1
Non
-PBD
EPB
EB0.01
0nd
18nd
–0.6
801.3±
0.91
BEHTB
P0.11
0nd
645.4±
1.7
40nd
–13
Dec
602
0.08
0nd
552.6±
1.0
5023
.7±
20.1
Dec
604CB
0.01
0nd
825.9±
2.5
8013
9±
130
Chlorden
ePlus
0.05
0nd
911.2±
0.2
808.7±
7.3
syn-Dechloran
ePlus
0.12
0nd
36nd
–9.1
904.4±
2.5
anti-Dechloran
ePlus
0.05
0nd
45nd
–2.8
801.8±
1.1
∑DP
6.3±
3.5
HBB
0.01
0nd
93.2
201.4–
3.9
DBD
PE1.21
0nd
926
.70
nd∑
Non
-PBD
Es15
.1±
4.3
179±
162
PBDE
∑PB
DEs
963±
126
2876
±53
338
73±
2260
nd=
notd
etected.
Not
detected
infish
:BDE-3,
-171
,-20
3,-205
,-20
6,an
d-208
aswella
soc
tabrom
o-1,3,3-trim
ethy
l-1-ph
enylinda
n(O
BIND),1,2-bis(2,4,6,-tribrom
ophe
noxy
)ethan
e(B
TBPE
),2-ethy
lhex
yl2,3,4,5-tetrab
romob
enzo
ate(EHTB
B),and
Dec
603.
Polybrom
inated
diph
enyl
ethe
r(PBD
E),p
entabrom
oethylbe
nzen
e(PBE
B),b
is(2-ethylhe
xyl)-tetrabrom
ophtha
late
(BEH
TBP),D
echloran
e60
2(D
ec60
2),D
echloran
e60
4Co
mpo
nent
B(D
ec60
4CB
),pe
ntab
romotolue
ne(PBT
),he
xabrom
oben
zene
(HBB
),2,2′,4,4,5,5′-he
xabrom
obiphe
nyl(BB
-153
).
51M. Houde et al. / Science of the Total Environment 479–480 (2014) 48–56
transformed to lipid weight basis (l.w.) in order to compare to otherpublished data. Wet weight (w.w.) concentrations of individualcongeners can be found in SI Table 1. Statistical analysis was performedusing JMP Statistics software and SAS (SAS Institute Inc., USA). Contami-nation level comparisonsweremade,within species, between fish of sim-ilar size, weight and body condition. Spearman correlations with Sidakstep-down analyses for multiplicity were used to evaluate associationsbetween fish morphological characteristics and to establish relationshipsbetween chemical tissue concentrations and lipid content. ANOVA wasused to compare chemical concentrations in perch between sites. Non-parametricWilcoxon testswere used to compare contaminant concentra-tions between species at the same sampling sites. The level for signifi-cance was set at p ≤ 0.05.
3. Results and discussion
Similarities in fish length, weight and body condition were requiredto appropriately compare chemical tissue concentrations betweensampling sites for each fish species. Results indicated that Fulton's con-dition factors were significantly related to extractable lipid percentagesin perch. Perch from Du Nord River had higher condition index thanperch from the St. Lawrence River sites (i.e., Boucherville and Beauregardislands, and Ilet Vert; p≤ 0.02) and had significantly higher lipid contentthan all other locations (p b 0.05). Perch from the L'Assomption Riverwere also significantly heavier than those from the other sites. Conse-quently, perch samples from Du Nord and L'Assomption Rivers wereexcluded from inter-site comparisons in PBDE tissue concentrations.Moreover, northern pike varied in size and weight between the threesampling sites with specimens collected in the Mille Iles River being sig-nificantly smaller than those from the Lake Deux-Montagnes (p b 0.01).Percentages of lipids in liver were also higher in Mille Iles pike comparedto Ilet Vert (p b 0.02). Results indicated similarities in weight, size andbody condition of pike between Lake des Deux-Montagnes and Ilet Vertenabling inter-site comparisons. The different tissues analyzed in fish(liver vs whole fish homogenate) complicated interpretation of data.Nevertheless, results enabled the identification and quantification ofemerging FRs in St. Lawrence River fish.
3.1. Emerging flame retardants
For thefirst time, Dec 604 CBwas quantified in fish species. A total of12 emerging non-PBDE FRswere detected and eight could be quantified(i.e., detected in b50% of individuals in each species) in liver of northernpike and muskellunge (Table 2). Non-PBDE concentrations were higherin muskellunge except for BEHTBP which was quantified in pike only.Differences in concentrations between species were however notstatistically significant (p N 0.05). The higher detection frequenciesof non-PBDE FRs in muskellunge could result from their higher trophicposition, and/or their larger habitat (and exposure) range. EmergingFRs were not detected in any yellow perch homogenates. This couldbe due to the lower trophic position of the species, the selected tissueused for analysis and its lower lipid content, and/or the younger ageof perch. Non-PBDE concentrations in fish liver were similar betweennorthern pike and muskellunge with concentrations of Dec 604 CBN BDE-183/Dec 604 N Dec 602 N CP (Fig. 2; p N 0.05). Contribution ofDec 604 was qualitatively minor compare to BDE-183 in the co-elution. Moreover, HBB was detected in liver of two muskellunge (1.4and 3.9 ng/g l.w.) and one northern pike (3.2 ng/g l.w.). OBIND,BTBPE, and EHTBBwere consistently below theMLOD in anyfish samplesfrom the St. Lawrence River and its tributaries.
3.2. Dechloranes
Both DP isomers could be quantified in liver of muskellunge withsyn-DP as the dominant one. Concentrations ranged from 0.5 to4.5 ng/g l.w. except for a highly contaminated muskellunge in Lake
Fig. 2. Concentrations (ng/g l. w.) of emerging FRs in northern pike and muskellunge collected in the St. Lawrence River and its tributaries. PBEB: pentabromoethylbenzene, BEHTBP:bis(2-ethylhexyl)-tetrabromophthalate, CP: Chlordene Plus, Dec: Dechlorane, Dec 604 CB: Dechlorane Component B, DP: Dechlorane Plus.
52 M. Houde et al. / Science of the Total Environment 479–480 (2014) 48–56
St-Louis (37.4 ng/g l.w.). These concentrations were higher than thosepreviously reported in whole walleye, whitefish, emerald shiner,white sucker, and goldeye from the south basin of Lake Winnipeg(0.04–0.82 ng/g l.w.) (Tomyet al., 2007b),muscle of yellow eels collect-ed from the St. Lawrence River and Lake Ontario (1.4 ± 0.95 and 0.9 ±0.41 ng/g l.w. respectively) (Sühring et al., in press), silver eel from LakeOntario (67± 48 pg/g l.w.) (Byer, 2013), and archivedwalleye samples(tissue not specified) from Lake Erie, Canada (0.14–0.91 ng/g l.w.) (Hohet al., 2006). Muskellunge concentrations thus correspond to thehighest values reported to date in fish from Canadian waters. Resultsfrom muskellunge may indicate point source contamination for DP up-stream or surrounding the greater Montreal area. DP concentrationswere within the range of those reported in muscle of common mullet,oriental goby, steed barbell, temperate sea bass, and crucian carpfrom Korea (0.61–126 ng/g l.w.) (Kang et al., 2010) and musclesamples pooled by species and sites for crucian carp, grasscarp, sharpbelly, common carp, weather loach from Liaohe River,Northeast China (8.7–93 ng/g l.w.) (Ren et al., 2013). Present resultsprovided additional evidence that DP is bioavailable in aquaticenvironments.
Other dechlorane compounds (i.e., Dec 602, 603, 604) have been re-ported in sediment (Shen et al., 2011a,b) although very limited data existfor these chemicals in biota andmore specifically fish (Table 3). Dec 602,
Table 3Dechlorane concentrations (ng/g l.w.) reported in fish worldwide.
Species Location Tissue Dec
Eelpout Northeast China Not specified 8.0European eel (yellow eel) Germany (Elbe) Muscle nd–European eel (silver eel) Germany (Elbe) Muscle 0.0European eel (silver eel) Germany (Rhine) Muscle 0.3American eel (yellow eel) Upper St. Lawrence River, Canada Muscle 1.2American eel (yellow eel) Lake Ontario Muscle 12.American eel (adult female) Lake Ontario Whole 0.8Lake trout and whitefish U.S./Canada Muscle 0.4Northern pike Canada Liver 2.6Muskellunge Canada Liver 24
nd = non-detected, na = not analyzed.LOD = limit of detection.
a Co-elution with BDE-183.b Mean range for samples collected in 1988, 1998, and 2008.
604 (which co-elutedwith BDE-183) and 604 CBwere found in pike andmuskellunge liver from the St. Lawrence River and this represents thefirst report of 604 CB in any species. Dec 603 was not detected in anySt. Lawrence River fish. Comparisons between northern pike of similarsize and body condition indicated that Dec 602 and Dec 604 CB concen-trations were significantly higher in the liver of pike collected at Ilet Vertcompared to Lake Deux-Montagnes (p N 0.02). Ilet Vert is located down-stream of Montreal in a section of the river where waters converge fromthe Mille Iles and the St. Lawrence Rivers. Both rivers receive effluentsfrom municipal effluents including the outfall of the large Montreal'sprimary WWTP (Fig. 1).
The range of Dec 602 concentrations in liver of muskellunge andnorthern pike from the St. Lawrence River were within levels reportedin Great Lakes fish muscle samples, collected near the only knowndechlorane production facility in North America, (Shen et al., 2010)and higher that levels reported in eels from Lake Ontario (Byer, 2013)and adult eels from the Upper St. Lawrence River (Sühring et al., inpress) (Table 3). Concentrations were also higher than levels found inNortheast China eelpout (Wang et al., 2012), European eel muscle(Sühring et al., 2013), and dolphin livers from Brazil (0.38 ng/g l.w.)(de la Torre et al., 2012). Dec 602 and Dec 603 were recently quantifiedin Chinese sturgeon eggs indicating the maternal transfer of thesechemicals (Peng et al., 2012).
602 Dec 603 Dec 604 Dec 604 CB Ref.
± 4.5 3.8 ± 2.1 nd na Wang et al. (2012)0.73 nd na na Sühring et al. (2013)6 ± 0.03 nd na na Sühring et al. (2013)0 ± 0.26 nd–0.37 na na Sühring et al. (2013)–11.4 0.57 ± 0.32 nd na Sühring et al. (in press)9–5.7 0.67 ± 0.07 nd na Sühring et al. (in press)8–1.9 0.01–0.02 0.002–0.02 na Byer (2013)7–34 0.014–0.55 0.06–1.3 na Shen et al. (2010)± 1.0 nd 4.0 ± 1.3a 5.9 ± 2.5b This study± 20 nd 28.7 ± 25.7a 139 ± 130
53M. Houde et al. / Science of the Total Environment 479–480 (2014) 48–56
Dec 604 CB was unexpectedly the predominant dechlorane com-pound in St. Lawrence fish. This first report of Dec 604 CB in aquaticbiota indicates that organisms are exposed to this chemical for whichexact sources remain unclear. It is presently unknown if Dec 604 CB isa degradation (debromination) product of Dec 604; their chemicalstructure is identical with the difference of one additional bromine forDec 604. Concentrations of BDE-183/Dec 604 in muskellunge and pikewere higher than levels reported in Great Lakes fish muscle samples(Shen et al., 2010). The high chlorination and the chemical propertiesof dechloranes indicate potential for persistence and bioaccumulationin the aquatic environment. Dec 602, BDE-183/Dec 604 and Dec 604CB concentrations in muskellunge liver samples were, in fact, higherthan DP levels, a pattern also observed in fish from the Great Lakes(Dec 602 N DP) (Shen et al., 2010). Spatial trends for Dec 602 and 604in sediment andfish indicate thatmanufacturing plants along theNiagaraRiver upstream of Lake Ontario were important sources of Dec 602 and604 to the Great Lakes, while Dec 603 in this region is likely from atmo-spheric deposition (Shen et al., 2010). The fact that Dec 604 has been re-ported in Great Lakes sediments and St. Lawrence River fish (presentstudy; co-elution with BDE-183), but not in any water, sediment, orbiota samples from China (Jia et al., 2011; Ma et al., 2012; Ren et al.,2013; Sun et al., 2013) or in dolphins from Brazil (de la Torre et al.,2012) suggest local emission sources of Dec 604 in North America. Addi-tional data in air and water samples could help understand environmen-tal sources and transport for dechloranes.
This information, in addition to the quantification of Dec 602,BDE-183/Dec 604, and Dec 604 CB in liver of apex pike and muskel-lunge, warrants further investigation on the environmental fate,biomagnification, and possible toxicological effects of dechloranesin aquatic organisms.
3.3. Chlordene Plus
The detection of CP in the liver of muskellunge and northern pikecollected from the St. Lawrence River adds information to previousreports of CP in biota in Lake Ontario lake trout (320–830 pg/g l.w.;samples from 1979 to 1993) (Shen et al., 2011a) and liver of franciscanadolphin from Brazil (nd–0.24 ng/g l.w.) (de la Torre et al., 2012). Thiscompound has also recently been detected in sediments from a numberof Great Lakes tributaries (Shen et al., 2011b). Chlordene Plus (CP) can bean impurity in technical Chlordene and Chlordane, although very littleinformation is available on its past and present production and use, envi-ronmental distribution and fate as well as toxicological effects. A declinein CP concentrations in suspended sediment has been observed in theNiagara River over the period 1980–2006 (Shen et al., 2011a) indicatingthat CP is not necessarily an emerging chemical but has been used in thepast without being studied in aquatic environments. The non-detectionof CP in yellow perch homogenate could be related to several factorssuch as fish age, trophic position, and the tissue analyzed. The highfrequency detection (N80%) of CP in St. Lawrence fish samples, atconcentrations up to 73 ng/g l.w., strongly suggested the occurrence ofproximate sources of this chemical in the studied aquatic environment.
3.4. PBEB
PBEB was an additive FR used in the 1970s and 1980s in thermosetpolyester and thermostatic resins. It has been detected in air samplesfrom Illinois, U.S. (Hoh et al., 2006) and Ontario, Canada (Gouteuxet al., 2008). In the present study, PBEBhas been detected in St. LawrenceRiver muskellunge and pike. Frequency detection and concentrations ofPBEB were both higher in muskellunge (80% of detection; 1.3 ±0.91 ng/g l.w.) than in pike (18% of detection; range: nd–0.6 ng/g l.w.)suggesting an increase with age and/or higher trophic position. Thelimited sample size did not allow inter-site comparisons for samplinglocations in the St. Lawrence River and tributaries. Elsewhere, PBEBwas found in muscle of walking catfish from South China (mean
concentration: 1.6 ng/g l.w.) (Zhang et al., 2010), lake trout from LakeOntario (17–320 ng/g l.w.) (Ismail et al., 2009), herring gull eggs fromthe Great Lakes basin (Gauthier et al., 2007), egg yolk of glaucous gullfrom the Norwegian Arctic (Verreault et al., 2007), harbor sealsfrom San Francisco Bay (0.07–0.5 ng/g l.w.) (Klosterhaus et al.,2012) and hooded seal (b0.02–3.7 ng/g l.w.) and right whales(b0.11–6.7 ng/g l.w.) from the Northwest Atlantic Ocean (Montieet al., 2010). The quantification of PBEB in muskellunge, and in otheraquatic organisms worldwide, in addition to reported bioaccumulationpotential (Wu et al., 2011) and the constant concentrations of PBEB infish tissue through time (1979–2004) (Ismail et al., 2009) highlightthe need to monitor this chemical in the environment as well as to in-vestigate its potential toxicity in aquatic organisms.
3.5. BEHTBP
BEHTBP was detected in 40% of muskellunge liver (range: nd–13 ng/g l.w.) and 64% of northern pike liver (range: nd–15.7 ng/g l.w.)collected in the St. Lawrence River locations nearMontreal. BEHTBP con-centrations found were higher than level previously reported in wholecapelins from the Arctic Ocean (719 ± 292 pg/g w.w.) (Sagerup et al.,2010) and European eels (elvers only, mean concentration of0.1 ng/g w.w.) from Germany (Sühring et al., 2013). BEHTBP is oneof two brominated chemicals integrated in Firemaster 550, FiremasterBZ-54, and DP-45 commercialized in 2003 as a replacement to penta-BDE in polyvinylchloride foam and plasticizer (Covaci et al., 2011).This FR has been detected in house dust at levels similar or lower thanthose of PBDEs (Stapleton et al., 2008; Shoeib et al., 2012) as well asin sewage sludge from WWTPs from the San Francisco Bay area(Klosterhaus et al., 2012) and blubber samples of Indo-Pacific hump-back dolphins and finless porpoises from China (Lam et al., 2009).Genotoxicity has been reported for BEHTBP in liver cells of fatheadmin-nows (Bearr et al., 2010) in addition to accumulation in whole fish. Forbirds, Gentes et al. (2012) recently reported BEHTBP in liver of urban-feeding omnivorous ring-billed gulls breeding on islands next to pres-ent fish sampling sites. The authors underlined the high frequency(89%) of BEHTBP in the St. Lawrence birds compared to other non-PBDE FRs screened for. Results for fish and gulls collected from thisdensely-populated metropolitan area, where large volumes of municipalWWTP effluents are released in the St. Lawrence River (see Fig. 1), indi-cate a need for abiotic/biotic analyses in order to identify sources of con-tamination and the environmental distribution of BEHTBP in this region.
3.6. PBDEs
PBDEs were the most abundant chemicals detected in St. LawrenceRiver fish. Out of the 38 PBDE congeners analyzed, BDE-3, -171, -203,-205, -206, and -208 were the only congeners not detected in any offish samples. Highly brominated BDE-207 and -209 were detected infish suggesting their bioavailability. The sum of PBDEs ranged between94 and 2480 ng/g l.w. in yellow perch, 1068 and 5971 ng/g l.w. in north-ern pike and 696 and 24,031 ng/g l.w. in muskellunge (Table 2, Fig. 3).PBDE concentrations detected in whole perch and liver of St. LawrenceRiver pike and muskellunge were among the highest reported levelsin top predatory fish from North America (see review by Shaw andKannan, 2009) and higher than concentrations reported in fish speciesfrom other St. Lawrence locations (i.e., American eels, rainbow smelt,and Atlantic tomcod) (Law et al., 2003; Byer et al., 2013). These resultssuggest point source of PBDE emission in the greater Montreal area.These findings corroborate data from a spatial study conducted in2001–2002 in the St. Lawrence River which revealed that PBDE concen-trations in eggs of Great blue heron around the island of Montreal wereup to ten-fold higher than measurements at other upstream or down-stream sites (Champoux et al., 2010). Results in these birds and inpresent fish were consistent with high levels of PBDEs previously
Fig. 3. Mean sum of PBDE and non-PBDE concentrations (and standard error) detected in yellow perch, northern pike and muskellunge collected from the St. Lawrence River.
54 M. Houde et al. / Science of the Total Environment 479–480 (2014) 48–56
reported in suspended particulate matters discharged at the Montreal'sWWTP (Pelletier and Rondeau, 2013).
Comparisons between sites for northern pike andmuskellungeweredifficult because of the low sample numbers. However, given largersample sizes, and since length, weight, and Fulton's condition factorsof perch did not differ statistically between the four St. Lawrence Riversites (i.e., Beauregard and Boucherville islands, Ilet Vert and Mille IlesRiver) comparisons in tissue concentrations between sites were pos-sible. Perch from Boucherville islands were the least contaminated(range: 95–247 ng/g l.w.) with PBDEs (p b 0.0001) while individualsfrom the Mille Iles River were the most contaminated ones (range:1423–1773 ng/g l.w., p b 0.0009). These results suggest the presenceof potential PBDE sources upstream or in the Mille Iles River which re-ceives waters from the Ottawa River and Lake des Deux Montagnes inaddition to numerous effluent outfalls. Dominant PBDE congeners andthe range of concentrations in pike and perch were similar to otherSt. Lawrence River data reported for these species (Laliberté, 2011).
Significant positive correlations were found between PBDE concen-trations and lipid percentages in perch homogenates (p b 0.05) which
0
10
20
30
40
50
Rel
ativ
e co
ntri
buti
on t
o ∑
PB
DE
(%
)
Yellow pe
Fig. 4. Relative contribution of individual PBDE congeners (%) to ∑PBDE concentrations (ng/g(liver) from the St. Lawrence River and its tributaries.
is in agreement with the octanol–water partition coefficients for thesecompounds (range: 5.7–8.3). These positive correlations were notobserved in the other fish species for which liver was analyzed. Concen-trations of individual congeners (BDE-17, -28, -47, -49, -99, -100, -126,-153, -154) detected in all fish were significantly different betweenthe three species (p b 0.01) with concentrations in perch b pikeb muskellunge. The predominant PBDE congeners in St. Lawrence fishwere BDE-47, -99, -100, -49, and -153 with the order varying betweenspecies (Fig. 4). Sampling location, trophic position, and diet could inpart explain the different patterns of contamination of these mostbioaccumulative PBDEs. The patterns were similar to those observedin fish from the North Sea (Boon et al., 2002), South China (Xianget al., 2007), and numerous sites in North America (reviews by Hites,2004 and Shaw and Kannan, 2009). The predominance of BDE-47 ob-served in yellow perch and northern pike was also found in Europeanflounders, soles, and eels (Bragigand et al., 2006), Arctic fish (reviewby de Wit et al., 2006), and weever, catfish, bartail flathead, wolfish,white flower croaker, and mullet sampled in Bohai Bay, North China(Wan et al., 2008). Results from St. Lawrence fish were consistent
rch Northern pike Muskellunge
l.w.) in yellow perch (whole fish homogenates), northern pike (liver) and muskellunge
55M. Houde et al. / Science of the Total Environment 479–480 (2014) 48–56
with the use of BDE-47, -99, -100 and -153 asmajor constituents of thepenta- and octa-BDE formulations which were extensively used inNorth America. These bioaccumulative congeners may also be biodeg-radation products of higher brominated PBDEs (Roberts et al., 2011).BDE-209 was detected in 60% of muskellunge liver samples, 36% ofpike samples and 21% of perch samples. These concentrationswere sim-ilar to levels reported in sea bassmuscle from Japan (0.4–0.81 ng/g l.w.)(Akutsu et al., 2001), whole cod from Norway (0.05–0.42 ng/g l.w.)(Sørmo et al., 2006) and lower than levels reported in carp and babelcollected in Spain (80 and 196 ng/g l.w.) (Eljarrat et al., 2007) andthree species of fish from Lake Michigan (0.18–1.2 μg/g l.w.) (Kuoet al., 2010). High concentrations (mean: 57 ± 12 ng/g w.w.) ofBDE-209 were found in ring-billed gulls nesting in the same geographi-cal region (Gentes et al., 2012). BDE-209was also reported in suspendedmatters downstream the Montreal's WWTP which was suggested as acontamination source (Pelletier and Rondeau, 2013). This suggests thebioavailability of BDE-209 in this urbanized area of the St. Lawrenceand the bioaccumulation potential of this fully brominated congener.
In conclusion, twelve emerging FRs, including the newly detectedDec 604 CB, and high concentrations of PBDEs, were detected in liver ofSt. Lawrence River top predatory fish. Results confirms the bioavailabilityof emerging FRs in human-impacted aquatic ecosystems and warrantscontinued environmental monitoring and toxicity testing for theseless-studied and novel FRs. Better understanding the toxicokinetics, en-vironmental fate, and ecotoxicological effects of these not-so-emergingsubstances is essential, if not critical, as their commercialization and useis likely to grow in the future.
The complete list of flame retardants analyzed in fish tissue, limits ofdetection (MLOD),meanmethod blank values, concentrations expressedon a wet weight basis and individual PBDE congeners expressed on alipid weight basis. This material is available free of charge. Supplemen-tary data associated with this article can be found, in the online version,at http://dx.doi.org/10.1016/j.scitotenv.2014.01.105.
Conflict of interest
No conflicts of interest to declare.
Acknowledgments
The authorswould like to thank Pierre Gagnon for data analyses andFrançois Boudreault for graphic work. Mélanie Douville, Simon-PierreDespatie, Claude Lessard, Christian Blaise, Timothée Debenest, andSamantha Tremblay (Centre St-Laurent, Environment Canada) andPhilippe Baillargeon, Guillaume Desrosiers, Sylvie Legendre, and RenéTherreult (MDDEFP) are thanked for their help during field work.Muskellunge fishermen are acknowledged for the fish collection. Wewould also like to thankVickyDoré and LingWang (Université duQuébecà Montréal) for their chemical analyses. This study was funded by Envi-ronment Canada's Chemical Management Plan (M.H.) and supports theSt. Lawrence Action Plan. Method development was possible throughthe Grants and Contribution program (to J.V.).
References
Akutsu K, Obana H, Okihashi M, Kitagawa M, Nakazawa H, Matsuki Y, et al. GC/MS analysisof polybrominated diphenyl ethers in fish collected from the Inland Sea of Seto, Japan.Chemosphere 2001;44(6):1325–33.
Bearr JS, Stapleton HM, Mitchelmore CL. Accumulation and DNA damage in fathead min-nows (Pimephales promelas) exposed to 2 brominated flame-retardant mixtures,firemaster® 550 and firemaster® BZ-54. Environ Toxicol Chem 2010;29(3):722–9.
Boon JP, Lewis WE, Tjoen-A-Choy MR, Allchin CR, Law RJ, De Boer J, et al. Levels ofpolybrominated diphenyl ether (PBDE) flame retardants in animals representing dif-ferent trophic levels of the North Sea food web. Environ Sci Technol 2002;36(19):4025–32.
Bragigand V, Amiard-Triquet C, Parlier E, Boury P, Marchand P, El Hourch M. Influence ofbiological and ecological factors on the bioaccumulation of polybrominated diphenylethers in aquatic food webs from French estuaries. Sci Total Environ 2006;368(2–3):615–26.
Byer JD. Organohalogenated persistent organic pollutants in American eels (Anguillarostrata) captured in eastern Canada [Ph.D. Thesis]Kingston, ON, Canada: Departmentof Chemistry, Queen's University; 2013.
Byer JD, Lebeuf M, Alaee M, Stephen BR, Trottier S, Backus S, et al. Spatial trends oforganochlorinated pesticides, polychlorinated biphenyls, and polybrominateddiphenyl ethers in Atlantic Anguillid eels. Chemosphere 2013;90(5):1719–28.
Cequier E, Marcé RM, Becher G, Thomsen C. Determination of emerging halogenatedflame retardants andpolybrominated diphenyl ethers in serumbygas chromatographymass spectrometry. J Chromatogr A 2013;1310:126–32.
Champoux L, Moisey J, Muir DCG. Polybrominated diphenyl ethers, toxaphenes, and otherhalogenated organic pollutants in great blue heron eggs. Environ Toxicol Chem2010;29(2):243–9.
Chen D, Letcher RJ, Burgess NM, Champoux L, Elliott JE, Hebert CE, et al. Flame retardantsin eggs of four gull species (Laridae) from breeding sites spanning Atlantic to PacificCanada. Environ Pollut 2012;168:1–9.
Covaci A, Harrad S, Abdallah MA-E, Ali N, Law RJ, Herzke D, et al. Novel brominated flameretardants: a review of their analysis, environmental fate and behaviour. Environ Int2011;37(2):532–56.
Crosse JD, Shore RF, Jones KC, Pereira MG. Long term trends in PBDE concentrationsin gannet (Morus bassanus) eggs from two UK colonies. Environ Pollut 2012;161:93–100.
Darnerud PO. Toxic effects of brominated flame retardants inman and inwildlife. EnvironInt 2003;29(6):841–53.
de la Torre A, AlonsoMB, MartínezMA, Sanz P, Shen L, Reiner EJ, et al. Dechlorane-relatedcompounds in franciscana dolphin (Pontoporia blainvillei) from Southeastern andSouthern Coast of Brazil. Environ Sci Technol 2012;46(22):12364–72.
de Wit CA, Alaee M, Muir DCG. Levels and trends of brominated flame retardants in theArctic. Chemosphere 2006;64(2):209–33.
deWit CA, Herzke D, Vorkamp K. Brominated flame retardants in the Arctic environment— trends and new candidates. Sci Total Environ 2010;408(15):2885–918.
Dietz R, Rigét FF, Sonne C, Born EW, Bechshøft T, McKinney MA, et al. Three decades(1983–2010) of contaminant trends in East Greenland polar bears (Ursus maritimus).Part 2: brominated flame retardants. Environ Int 2012;59:494–500.
Eljarrat E, Labandeira A, Marsh G, Raldúa D, Barceló D. Decabrominated diphenyl ether inriver fish and sediment samples collected downstream an industrial park. Chemosphere2007;69(8):1278–86.
ESIS. European Chemical Substances Information System. http://esis.jrc.ec.europa.eu/.Feo ML, Barón E, Eljarrat E, Barceló D. Dechlorane Plus and related compounds in aquatic
and terrestrial biota: a review. Anal Bioanal Chem 2012;404(9):2625–37.Gauthier LT, Hebert CE,Weseloh DVC, Letcher RJ. Current-use flame retardants in the eggs
of herring gulls (Larus argentatus) from the Laurentian Great Lakes. Environ SciTechnol 2007;41(13):4561–7.
Gentes M-L, Letcher RJ, Caron-Beaudoin É, Verreault J. Novel flame Retardants inurban-feeding ring-billed gulls from the St. Lawrence River, Canada. Environ SciTechnol 2012;46(17):9735–44.
Gouteux B, Alaee M, Mabury SA, Pacepavicius G, Muir DCG. Polymeric brominated flameretardants: are they a relevant source of emerging brominated aromatic compoundsin the environment? Environ Sci Technol 2008;42(24):9039–44.
Guerra P, Alaee M, Jiménez B, Pacepavicius G, Marvin C, MacInnis G, et al. Emerging andhistorical brominated flame retardants in peregrine falcon (Falco peregrinus) eggsfrom Canada and Spain. Environ Int 2012;40(1):179–86.
Hites RA. Polybrominated diphenyl ethers in the environment and in people: ameta-analysis of concentrations. Environ Sci Technol 2004;38(4):945–56.
Hoguet J, Keller JM, Reiner JL, Kucklick JR, Bryan CE, Moors AJ, et al. Spatial and temporaltrends of persistent organic pollutants andmercury in beluga whales (Delphinapterusleucas) from Alaska. Sci Total Environ 2013;449:285–94.
Hoh E, Zhu L, Hites RA. Dechlorane Plus, a chlorinated flame retardant, in the Great Lakes.Environ Sci Technol 2006;40:1184–9.
Howard PH, Muir DCG. Identifying new persistent and bioaccumulative organics amongchemicals in commerce. Environ Sci Technol 2010;44(7):2277–85.
Ismail N, Gewurtz SB, Pleskach K, Whittle M, Helm PA, Marvin CH, et al. Brominated andchlorinated flame retard ants in Lake Ontario, Canada, Lake Trout (Salvelinusnamaycush) between 1979 and 2004 and possible influences of food-web changes.Environ Toxicol Chem 2009;28(5):910–20.
Jia H, Sun Y, Liu X, Yang M, Wang D, Qi H, et al. Concentration and bioaccumulation ofdechlorane compounds in coastal environment of Northern China. Environ SciTechnol 2011;45(7):2613–8.
Kang J-H, Kim J-C, Jin G-Z, Park H, Baek S-Y, Chang Y-S. Detection of Dechlorane Plus infish from urban-industrial rivers. Chemosphere 2010;79:850–4.
Klosterhaus SL, Stapleton HM, La Guardia MJ, Greig DJ. Brominated and chlorinatedflame retardants in San Francisco Bay sediments and wildlife. Environ Int2012;47:56–65.
Kuo Y-M, Sepúlveda MS, Hua I, Ochoa-Acuña HG, Sutton TM. Bioaccumulation andbiomagnification of polybrominated diphenyl ethers in a food web of Lake Michigan.Ecotoxicology 2010;19(4):623–34.
Laliberté D. Teneurs en polybromodiphényléthers (PBDE) dans les poissons du fleuveSaint-Laurent et des lacs et rivières du Québec (2002–2008). Québec, Ministère duDéveloppement durable, de l'Environnement et des Parcs, Direction du suivi de l'étatde l'environnement; 2011 [48 pp.].
Lam JCW, Lau RKF, Murphy MB, Lam PKS. Temporal trends of hexabromocyclodecanes(HBCDs) and polybrominated diphenyls ethers (PBDEs) and detection of two novelflame retardants in marine mammals from Hong Kong, South China. Environ SciTechnol 2009;43:6944–9.
Law RJ, Alaee M, Allchin CR, Boon JP, Lebeuf M, Lepom P, et al. Levels and trends ofpolybrominated diphenylethers and other brominated flame retardants in wildlife.Environ Int 2003;29(6):757–70.
56 M. Houde et al. / Science of the Total Environment 479–480 (2014) 48–56
Law K, Halldorson T, Danell R, Stern G, Gewurtz S, Alaee M, et al. Bioaccumulation andtrophic transfer of some brominated flame retardants in a Lake Winnipeg (Canada)food web. Environ Toxicol Chem 2006;25(8):2177–86.
Ma J, Qiu X, Zhang J, Duan X, Zhu T. State of polybrominated diphenyl ethers in China: anoverview. Chemosphere 2012;88(7):769–78.
Möller A, Xie Z, Sturm R, Ebinghaus R. Large-scale distribution of Dechlorane Plus in airand seawater from the Arctic to Antarctica. Environ Sci Technol 2010;44:8977–82.
Montie EW, Letcher RJ, Reddy CM, Moore MJ, Rubinstein B, Hahn ME. Brominated flameretardants and organochlorine contaminants in winter flounder, harp and hoodedseals, and North Atlantic right whales from the Northwest Atlantic Ocean. Mar PollutBull 2010;60:1160–9.
Munschy C, Héas-Moisan K, Tixier C, Boulesteix L, Morin J. Classic and novel brominatedflame retardants (BFRs) in common sole (Solea solea L.) from main nursery zonesalong the French coasts. Sci Total Environ 2011;409(21):4618–27.
Pelletier M, Rondeau M. Polybrominated diphenyl ethers in the suspended matter andsediments of the St. Lawrence River. I. Report: 978-1-100-20752-0. Canada: Ministerof the Environment; 2013. [12 pp.].
Peng H, Zhang K, Wan Y, Hu J. Tissue distribution, maternal transfer, and age-related ac-cumulation of Dechloranes in Chinese Sturgeon. Environ Sci Technol 2012;46:9907–13.
Ren G, Wang Z, Yu Z, Wang Y, Ma S, Wu M, et al. Primary investigation on contaminationpattern of legacy and emerging halogenated organic pollutions in freshwater fishfrom Liaohe River, Northeast China. Environ Pollut 2013;172:94–9.
Roberts SC, Noyes PD, Gallagher EP, Stapleton HM. Species-specific differences andstructure–activity relationships in the debromination of PBDE congeners inthree fish species. Environ Sci Technol 2011;45(5):1999–2005.
Sagerup K, Herzke D, Harju M, Evenset A, Christensen GN, Routti H, et al. New brominatedflame retardants in Arctic biota. Tromsø: KLIF; 2010 [30 pp.].
Shaw SD, Kannan K. Polybrominated diphenyl ethers in marine ecosystems of theAmerican continents: foresight from current knowledge. Rev Environ Health2009;24(3):157–229.
Shen L, Reiner EJ, Macpherson KA, Kolic TM, Sverko E, Helm PA, et al. Identificationand screening analysis of halogenated norbornene flame retardants in theLaurentian Great Lakes: Dechloranes 602, 603, and 604. Environ Sci Technol2010;44(2):760–6.
Shen L, Reiner EJ, Helm PA, Marvin CH, Hill B, Zhang X, et al. Historic trends of Dechloranes602, 603, 604, Dechlorane Plus and other norbornene derivatives and their bioaccumu-lation potential in Lake Ontario. Environ Sci Technol 2011a;45(8):3333–40.
Shen L, Reiner EJ, Macpherson KA, Kolic TM, Helm PA, Richman LA, et al. Dechloranes602, 603, 604, Dechlorane Plus, and Chlordene Plus, a newly detected analogue,in tributary sediments of the Laurentian Great Lakes. Environ Sci Technol2011b;45:693–9.
Shen L, Jobst KJ, Helm PA, Reiner EJ, McCrindle R, Tomy GT, et al. Identification and deter-mination of the dechlorination products of Dechlorane 602 in Great Lakes fish andArctic beluga whales by gas chromatography-high resolution mass spectrometry.Anal Bioanal Chem 2012;404:2737–48.
Shoeib M, Harner T, Webster GM, Sverko E, Cheng Y. Legacy and current-use flame retar-dants in house dust from Vancouver, Canada. Environ Pollut 2012;169:175–82.
Siddique S, Xian Q, Abdelouahab N, Takser L, Phillips SP, Feng Y-L, et al. Levels ofdechlorane plus and polybrominated diphenylethers in human milk in two Canadiancities. Environ Int 2012;39(1):50–5.
Sørmo EG, Salmer MP, Jenssen BM, Hop H, Bæk K, Kovacs KM, et al. Biomagnification ofpolybrominated diphenyl ether and hexabromocyclododecane flame retardants inthe polar bear food chain in Svalbard, Norway. Environ Toxicol Chem 2006;25(9):2502–11.
Stapleton HM, Allen JG, Kelly SM, Konstantinov A, Klosterhaus S, Watkins D, et al. Alter-nate and new brominated flame retardants detected in US home dust. Environ SciTechnol 2008;42:6910–6.
Sühring R, Möller A, Freese M, Pohlmann J-D, Wolschke H, Sturm R, et al. Brominatedflame retardants and dechloranes in eels from German Rivers. Chemosphere2013;90:118–24.
Sühring R, Byer J, Freese M, Pohlmann J-D, Wolschke H, Möller A, et al. Brominated flameretardants and Dechloranes in European and American eels from glass to silver lifestages. Chemosphere 2014. http://dx.doi.org/10.1016/j.chemosphere.2013.10.096.[in press].
Sun J, Zhang A, Fang L, Wang J, Liu W. Levels and distribution of Dechlorane Plus and re-lated compounds in surficial sediments of the Qiantang River in eastern China: the re-sults of urbanization and tide. Sci Total Environ 2013;443:194–9.
Sverko E, Tomy GT, Reiner EJ, Li Y-F, McCarry BE, Arnot JA, et al. Dechlorane Plus andrelated compounds in the environment: a review. Environ Sci Technol 2011;45:5088–98.
Tomy GT, Palace VP, Pleskach K, Ismail N, Oswald T, Danell R, et al. Dietary exposure ofjuvenile rainbow trout (Oncorhynchus mykiss) to 1,2-bis(2,4,6-tribromophenoxy)ethane: bioaccumulation parameters, biochemical effects, and metabolism. EnvironSci Technol 2007a;41(14):4913–8.
Tomy GT, Pleskach K, Ismail N, Whittle DM, Helm PA, Sverko E, et al. Isomers ofDechlorane Plus in Lake Winnipeg and Lake Ontario food webs. Environ Sci Technol2007b;41:2249–54.
Tomy GT, Thomas CR, Zidane TM, Murison KE, Pleskach K, Hare J, et al. Examination ofisomer specific bioaccumulation parameters and potential in vivo hepaticmetabolitesof syn- and anti-Dechlorane Plus isomers in juvenile rainbow trout (Oncorhynchusmykiss). Environ Sci Technol 2008;42:5562–7.
Verreault J, Gebbink WA, Gauthier LT, Gabrielsen GW, Letcher RJ. Brominated flameretardants in glaucous gulls from the Norwegian arctic: more than just an issueof polybrominated diphenyl ethers. Environ Sci Technol 2007;41(14):4925–31.
Wan Y, Hu J, Zhang K, An L. Trophodynamics of polybrominated diphenyl ethers in themarine food web of Bohai Bay, North China. Environ Sci Technol 2008;42(4):1078–83.
Wang D-G, Yang M, Qi H, Sverko E, Ma W-L, Li Y-F, et al. An Asia-specific source ofdechlorane plus: concentration, isomer profiles, and other related compounds. Envi-ron Sci Technol 2010;44(17):6608–13.
Wang L, Jia H, Liu X, Yang M, Hong W, Sun Y, et al. Dechloranes in a river in northeasternChina: spatial trends in multi-matrices and bioaccumulation in fish (Enchelyopuselongatus). Ecotoxicol Environ Saf 2012;84:262–7.
Wu J-P, Guan Y-T, Zhang Y, Luo X-J, Zhi H, Chen S-J, et al. Trophodynamics ofhexabromocyclododecanes and several other non-PBDE brominated flame retardantsin a freshwater food web. Environ Sci Technol 2010a;44(14):5490–5.
Wu J-P, Zhang Y, Luo XJ, Wang Y, Chen SJ, Guan YT, et al. Isomer-specific bioaccumulationand trophic transfer of Dechlorane Plus in the freshwater foodweb from a highly con-taminated site, South China. Environ Sci Technol 2010b;44:606–11.
Wu J-P, Guan Y-T, Zhang Y, Luo X-J, Zhi H, Chen S-J, et al. Several current-use, non-PBDEbrominated flame retardants are highly bioaccumulative: evidence from field deter-mined bioaccumulation factors. Environ Int 2011;37(1):210–5.
Xiang C-H, Luo X-J, Chen S-J, Yu M, Mai B-X, Zeng EY. Polybrominated diphenyl ethers inbiota and sediments of the Pearl River Estuary, South China. Environ Toxicol Chem2007;26(4):616–23.
Zhang Y, Luo X-J, Wu J-P, Liu J, Wang J, Chen S-J, et al. Contaminant pattern and bio-accumulation of legacy and emerging organhalogen pollutants in the aquaticbiota from an e-waste recycling region in South China. Environ Toxicol Chem2010;29(4):852–9.