Nanopore-based single-molecule DNA analysis
Transcript of Nanopore-based single-molecule DNA analysis
-
Ken HealyUniversity College Cork, Department of Electrical and Electronic Engineering, IrelandTel.: +353 214 902 944;Fax: +353 214 902 944;E-mail: [email protected]
ea of ana- protein
a mecha-to enter ated poly- obviouslyt until heton frompart of
Keywords: -hemolysin, DNA and RNA translocation, DNA sequencing, single-molecule analysis, synthetic and solid-state nanopores
trical detectors, together with nanotechnologi-cal engineering, to isolate individual moleculesfor detection. Comparative reviews of thesemethods can be found elsewhere [13].
Nanopore-based DNA analysis is one suchmethod, which is still at the research stage, andhas the potential to operate orders of magni-tude faster than current techniques. This is
nia, Davis (CA, USA), conceived the idlyzing DNA with nanometer-sizedchannels in 1989 while searching fornism to enable individual nucleotides liposome containing an encapsulamerase. However, in the absence of ansuitable channel, the idea lay dormandiscussed it in 1991 with Daniel BranNanopore-10.2217/17435889.2.4.459 2operates. Essential for these efforts are techniquesand instrumentation to analyze DNA.
A fundamental challenge in all DNA analysesis to bridge between our own macroscale andthe nanoscale at which these molecules exist.Current methods accomplish this by processingmillions or billions of molecules simultane-ously, so that the combined result is largeenough to be observed. But this adds prepara-tion time, cost and averages any variations. Sev-eral single-molecule methods are indevelopment that avoid these limitations byanalyzing individual molecules one at a time,relying on new, more sensitive, optical and elec-
results to date. The field also contains severaltheoretical analyses and simulation studies,although these are not covered here owing tospace limitations. Interested readers are directedto a review by Meller [10] and also to the growingbody of more recent work, which can be identi-fied by a search of the literature. In addition tonucleic acids, other molecules have also beenstudied through nanopore-based analysis [1118];however, this discussion will be limited to workinvolving DNA and RNA.
HistoryDavid Deamer, then at the University of Califor-REVIEW
based single-molecule DNA analysisNanopore-based DNA analysis is a single-molecule technique with revolutionary potential. It promises to carry out a range of analyses, orders of magnitude faster than current methods, including length measurement, specific sequence detection, single-molecule dynamics and even de novo sequencing. The concept involves using an applied voltage to drive DNA molecules through a narrow pore that separates chambers of electrolyte solution. This voltage also drives a flow of electrolyte ions through the pore, measured as an electric current. When molecules pass through the pore, they block the flow of ions and, thus, their structure and length can be determined based on the degree and duration of the resulting current reductions. In this review, I explain the nanopore-based DNA analysis concept and briefly explore its historical foundations, before discussing and summarizing all experimental results reported to date. I conclude with a summary of the obstacles that must be overcome for it to realize its promised potential.
The desire to understand life is perhaps a defin-ing characteristic of humanity. It is a key drivingforce in scientific research and associated tech-nology development. This search for under-standing has led to the molecular scale and thediscovery of nucleic acids and proteins as thefundamental recipes, machinery and buildingblocks for living organisms. Nucleic acids DNA in most cases hold instructions for everyaspect of organism behavior at the cellular level.Owing to this key role, huge research efforts havefocused, and continue to focus, on how DNA
highly desirable because it would enable thepower of DNA analysis to be exploited in amuch wider range of research and clinical set-tings. Nanopore-based DNA analysis isoutlined in Box 1.
This review joins several previous commentarieson the nanopore-based DNA analysis field [48]. Aset of laboratory protocols for single-moleculeDNA analysis with both biological and syntheticpores has also been prepared recently [9]. Thisreview updates past coverage but also presents aself-contained overview of all experimental007 Future Medicine Ltd ISSN 1743-5889 Nanomedicine (2007) 2(4), 459481 459
k.rowlandText BoxFor reprint orders, please contact:[email protected]
-
REVIEW Healy
460Harvard University (MA, USA), his formerpostdoctoral advisor. At this stage, they made apatent disclosure and, in the process, discov-ered that George Church, also at Harvard, haddisclosed a similar concept independently.Choosing to work together, they eventuallyreceived a patent on the common technique[301]. Still searching for a suitable channel,Church and his postdoctoral associate Bald-arelli began to work with a bacteriophagemotor protein, whereas Deamer contactedJohn Kasianowicz at the National Institute ofStandards and Technology (NIST), who wasworking with the -hemolysin channel, ofinterest owing to its much larger size compared
with usual biological channels. After positiveinitial tests, Kasianowicz, Deamer and Brantonassembled at NIST in February 1994 and con-vinced themselves they really were observingnucleic acid molecules passing through thepore. Deamer and Branton then soughtNational Science Foundation funding to sup-port continuation of the work, which resultedin their landmark 1996 paper [19]. This sum-mary was based on a personal communicationfrom David Deamer.
For the origins of this method, one has to goback to the 1940s, when Wallace Coulter wasattempting to standardize particle size in paintsfor the US Navy [201]. Having run out of paint
Box 1. Nanopore-based DNA analysis.
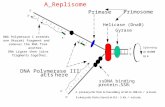
The concept of nanopore-based DNA analysis is surprisingly simple. As shown in Figure 1, an electrochemical system is used, in which an applied voltage drives a flow of ions through a nanopore. The DNA molecule to be analyzed is also driven through the pore by the applied voltage, blocking the flow of ions. This ion flow can be measured as a current flowing in the electrical circuit and, because the pore is chosen to have a diameter sufficiently small that DNA is forced to pass through as a linear strand (as opposed to the randomly coiled globule configuration adopted by DNA molecules in free solution), the molecules properties length and, ultimately, sequence can be determined based on the duration of current blockage and variations in its magnitude.
Figure 1. Nanopore-based DNA analysis.
The side where DNA is added is typically referred to as cis, with the other side trans. The DNA molecule and nanopore are not shown to scale in this diagram.
A
VBIAS
+
transTranslocation direction
cis transNanomedicine (2007) 2(4) future science groupfuture science group
-
Nanopore-based single-molecule DNA analysis REVIEW
future science groupfuture science groupon one occasion, he tried a sample of his ownblood and realized that red blood cells could besimilarly detected as they are driven by a pres-sure difference through a small aperture,restricting the flow of ions through that aper-ture as they pass. He patented the idea [302],later termed the resistive-pulse technique, andfounded Coulter Electronics with his brotherJoe to develop and market instruments forblood cell counting and cell sizing.
In the 1970s, DeBlois and Bean, at GeneralElectric, used track-etched pores to improvethe detection limit to approximately 60 nmand introduced the concept of electrophoreti-cally driving charged particles through thepore [20,21].
Meanwhile, the existence of biological nano-pores ion channels in cell membranes wasexplored from the 1940s onward by Hodgkin,Huxley and others, as recounted by Hille [22].Subsequently, supposed channel-forming pro-teins were applied to lipid bilayers, enablingtheir study under more controlled conditionsand demonstrating behavior strongly support-ing the existence of discrete channels [2326]. Inthe 1970s, Neher and Sakmann developed thepatch-clamp technique and associated sensitiveelectronics [27,28], which enabled them to con-firm the existence of discrete ion channelsdirectly [29].
Upon these foundations, Bezrukov et al. high-lighted the possibility of single-molecule detec-tion in ion channels by counting polyethyleneglycol (PEG) molecules passing through analamethicin ion channel based on the reducedconductance and increased noise that theyinduce [11]. This, together with the characteriza-tion [30] and crystal structure [31] of the -hemo-lysin channel, set the stage for nanopore-basedDNA analysis.
The nanopore-based DNA analysis field is nowturning back to its synthetic pore foundations, asfabrication methods approach the precisionnecessary to emulate these biological structures.
Research with biological poresThe initial work by Kasianowicz, Brandin,Branton and Deamer examined the interactionsof single-stranded (ss)RNA [poly(A/G/T/C)]and ssDNA [poly(dA/dG/dT/dC)] homo-polymers, 100450 nt in length, with the-hemolysin pore [19]. They observed charac-teristic transient reductions in current, only inthe presence of polynucleotides, and demon-
correspond to individual polynucleotide mole-cules translocating through the pore in alinear manner. This was based on severalresults:
The frequency of these transient events wasdirectly proportional to the concentration ofmolecules present;
The durations of these events were propor-tional to polynucleotide length and inverselyproportional to applied voltage;
Double-stranded (ds)DNA with overhangingsingle-stranded ends would frequently causeindefinite current reduction (as expectedbecause dsDNA is too large to fit through the-hemolysin pore), reversible by temporarilychanging the voltage polarity;
Competitive PCR analysis of the solution onthe trans side of the pore detected the trans-mitted DNA at a concentration matching thenumber of events observed (Figure 1 illustratesthe definitions of cis and trans in the contextof these experiments).
Similar translocations of dsDNA throughother biological channels (a bacterial ion chan-nel from Bacillus subtilis membrane vesicles [32]and the voltage-dependent anion channel [33])have also been confirmed by PCR analysis;however, in these cases, corresponding clearlydefined ion current events were not observed.The authors show that these channels exhibitgating behavior (i.e., fluctuations between twoor more conductance states), believed to be dueto mechanical motion of flexible sections in thepeptide chains of which they are composed.Their results also show that this gating behavioris enhanced when DNA is driven through thechannels, and speculate that gating obscures thewell-defined ion current reductions expectedfor translocating DNA.
Effects of polymer compositionSubsequently, Akeson et al. improved the exper-imental setup to enable lower noise levels andlower analysis volumes (Figure 2) and used it tocompare translocation of RNA homopolymerspecies [poly(A),poly(C),poly(U)] and also toexamine the RNA diblock copolymerpoly(A)30(C)70 [34]. They observed characteris-tic depths and durations for each homopolymerspecies (see example plots in Figure 3). In somecases, depth or duration alone was not sufficientto separate event classes, but a combination ofthe two gave a unique signature. Similar depth461www.futuremedicine.com
strated conclusively that these events variations in a single event were visible with the
-
REVIEW Healy
462
Figure 2. -hemolys
Setup used by Akeson et20-m diameter aperturebilayer as shown the secompared with the vestiOriginal figure courtesy o
-hem
+
diblock copolymer; however, the authors cau-tioned that this does not translate directly to theability to identify A and C in a randomsequence, based on their deduction that thesedepth differences are owing to different second-ary structures adopted by the homopolymerregions (Figure 4) rather than differences betweenthe individual nucleotides.
Subsequently, Meller et al. examined translo-cations of ssDNA homopolymers, two-baserepeat polymers [poly(dAdC)50,poly(dCdT)50]and a diblock copolymer [poly(dA)50(dC)50],all 100 nt in length [35]. They refined the clas-sification of events, summarizing each classuniquely using three statistical parameters: IP,the most probable event depth; tp, the mostprobable event duration; and T, the exponen-tial decay constant of the distribution of eventdurations. Using these parameters, they predictthat it is possible to classify most individualtranslocation events with high confidence.This is demonstrated for a mixture ofpoly(dA)100 and poly(dC)100, in which theyidentify 98% of 999 events unambiguously
Later, Khulbe et al. also investigated translo-cations of ssDNA diblock copolymers as proofof concept for a DNA-based high-density data-storage system [36], producing results similar tothose aforementioned by Akeson et al..
Temperature dependenceMeller et al. also added temperature control totheir experimental setup and examined the tem-perature dependence of event classes at 1540C[35]. As in the aforementioned work, they observedthat event classes for certain polynucleotides con-tained two distinct groups. The separation ofthese groups and the relative proportion of eventsin each can change with temperature.
For each polymer studied, the primarygroup closely followed a T-2 dependence, withall appearing to converge at high tempera-tures. As noted, this latter observation suggeststhat polymer lengths could be inferreddirectly, independent of sequence. This sug-gestion has yet to be tested. The secondarygroups showed more complex temperaturedependences for polymers containing contigu-
in experimental setup.
al. [34] and most subsequent researchers. A U-tube connects two small-volume chambers and is tapered to a on one side. This gives a small bilayer area and, thus, high resistance. An -hemolysin channel inserts in the ction of the channel embedded in the bilayer is referred to as the stem or barrel and has a narrower diameter bule region contained in the protruding cap. f M Akeson.
100 mV
+
1 M KCl70 l
20-m diameteraperture
olysin
2.0 nm
A
Nanomedicine (2007) 2(4) future science groupfuture science group
with 90% confidence. ous sequences of adenine. Similar to Akeson
-
Nanopore-based single-molecule DNA analysis REVIEW
future science groupfuture science groupet al., this was concluded owing to the second-ary structure that these sequences are knownto adopt and temperature variations of thissecondary structure.
Polynucleotide orientation dependenceContinuing this thinking, Meller et al. also con-cluded that the existence of primary and second-ary groups corresponded to polymers existing intwo different conformations, rather than the pol-ymers translocating in 53 and 35 direc-tions, as had been suggested previously. Recentresults and simulations show, however, that thelatter hypothesis is the dominant factor [3639].Also, later results by some of the authors dis-puted the impact of secondary structure on eventdurations, in favor of the interaction of purinebases (A or G) with the pore [40].
Subsequently, Butler et al. carried out adetailed study of RNA diblock copolymertranslocations, including separation of translo-cation event signals into mid- and transloca-tion-states (Figure 5) [39]. The additionalinformation provided by this analysis reinforcedtheir conclusion that 53 and 35 trans-locations of poly(C) segments give rise to differ-
The poly(C)50 and poly(A)25(C)50 moleculesalso showed a higher likelihood to translocatein the 53 direction.
Event frequencyHenrickson et al. examined the dependence ofevent frequency on driving voltage, finding anexponential relationship, with a threshold volt-age below which events did not occur, in agree-ment with a Vant HoffArrhenius model [41].This threshold was greater when polynucle-otides were placed on the narrower, barrel sideof the pore (Figure 2), which the authors sug-gested could be owing to the narrower con-striction on this side representing a higherentropic energy barrier or owing to electro-static attraction (repulsion) as a result of posi-tively (negatively) charged residues known tobe present on the wider (narrower) ends of thechannel, respectively. The authors also studieda similar condition, the threshold voltageneeded to hold a molecule in the pore againstthermal agitation and repulsive forces, by cap-ping a ssDNA with a biotinavidin linkage,which was large enough to prevent the mole-cule passing through the pore completely. The
Figure 3. Event depth versus duration plots for various single-stranded DNA and RNA homopolymers.
As observed by Butler et al. [39]. Different homopolymers have different distributions, some containing two recognizable clusters. Figure courtesy of TZ Butler.
Event duration (s)
I TS/I O
SI TS
/I OS
0
0.1
0.2
0
0.1
0.2
102 103 104 105 102 103 104 105 102 103 104 105
dA50 dC50 dT50
A50 C50 U50463www.futuremedicine.com
ing and distinguishable event characteristics. magnitude of threshold voltage required was
-
REVIEW Healy
464lower for capped ssDNA placed on the narrowside of the pore, as opposed to free ssDNAbehavior. They did not carry out further analy-sis but suggested that interactions between theavidin cap and the pore could be responsible.Restricting DNA movement with techniquessuch as this has been used widely and will bediscussed in more detail later in the section ontrapping DNA.
Meller et al. observed the same exponentialdependence over the 55120-mV region exam-ined by Henrickson et al., but measuring athigher voltages revealed a second regime withlower exponent [40]. These measurements weremade possible by conducting experiments atlow temperatures otherwise event durationswere too short to be measured accurately. Theyreasoned that, with increasing voltage, poly-nucleotides will become more concentratednear the pore, meaning that the VantHoffArrhenius model can no longer beapplied. The increased concentration limitscapture rate owing to the resulting intermolecu-lar collisions, repulsion and the fact that onlyone molecule can translocate at a time.
The dependence of event frequency on volt-age was predicted at even higher voltages byNakane et al., based on a derived capture rateR = 4 C D a P(V), where C is the polynucle-otide concentration, D its diffusion coeffi-cient, a the pore radius and P(V) theprobability that a polynucleotide that collideswith the pore entrance will then translocatethrough it [42]. P(V) was estimated based onMonte Carlo simulations.
Influence of polynucleotide lengthMeller et al. studied the effects of polymerlength on event depth and translocation veloc-ity, finding strong dependences for shortlengths and negligible dependences for longerlengths [43]. The transition point between thesecharacteristics was approximately 12 bases,which corresponds well with the length of the-hemolysin pore. Also, analysis of translo-cation-velocity voltage dependence, in the con-text of mathematical models [44,45], enabledupper bounds to be established on the energypenalty of the polymer extension required toenable translocation and on the reduced diffu-sion coefficient for ssDNA confined in the-hemolysin pore.
Identification of modified polynucleotidesThe effects of 5 and 3 phosphorylation (thepresence of leading [5] or trailing [3] phos-phate groups on a polynucleotide molecule) ontranslocation event characteristics was investi-gated by Wang et al. [37]. Their results showthat it is possible to detect and differentiatebetween all combinations of present or absentphosphorylation at either end, as a result oftheir finding that phosphorylation affects theease with which the relevant end of the mole-cule can enter the pore. They also demon-strated the sensitivity of the nanopore systemto polynucleotide degradation and purity,which can vary with manufacturing techniquesand laboratory processing, thus highlightingthe potential impact of low-qualityDNA/RNA samples.
Figure 4. Secondary structures expected for various DNA and RNA homopolymers, relative to the -hemolysin pore.
As described by Akeson et al. [34]. As can be seen, both poly(C) and poly(A) adopt helical structures, the latter of which appears too large to fit through the channel without unwinding.
Poly(C) Poly(A) Poly(dC)Nanomedicine (2007) 2(4) future science groupfuture science group
-
Nanopore-based single-molecule DNA analysis REVIEW
future science groupfuture science groupNew approachesIn 20002001, three offshoots grew from themain theme of examining unhindered transloca-tion of ssDNA/RNA through -hemolysin pores.These were analysis of DNA and RNA by trap-ping it in the pore using a variety of methods,application of genetically engineered mutant-hemolysin variants to provide new capabilitiesand experiments with synthetic nanopores.
Trapping DNAAlthough the typical speed of polynucleotidetranslocation through the -hemolysin pore isone of the potentially revolutionary advantagesof nanopore-based analysis, the resolution oftodays electronic detection methods limits theamount of information that can be gathered atthese speeds. Thus, an important approach hasbeen to restrict polynucleotide motion in vari-ous ways, so that it can be observed for alonger time.
All of the methods described hereafter restrictDNA motion by modifying either the DNA orthe pore; however, Jeon et al. have attempted adifferent approach recently: modifying the envi-ronment. They encapsulated the pore and lipidbilayer in a hydrogel with dense matrix, whichwas expected to significantly constrain themotion of DNA molecules, and provide a pre-liminary result that they believe shows DNAtranslocating two orders of magnitude slowerthan expected through an -hemolysin pore [46].If confirmed by a more detailed analysis, this
because its implementation is not costly orcomplicated and it could also be applied withsynthetic nanopores.
BiosensingTrapping DNA is also the basis for a genericnanopore-based biomolecule sensor. As previ-ously mentioned, Henrickson et al. were thefirst to adopt this approach, using ssDNA 5modified with a biotin group, which bindsstrongly to avidin proteins, resulting in acapped complex that cannot pass through thepore completely [41]. Kasianowicz et al. demon-strated a prototype biosensor based on this con-cept, in which the avidin either reduces thefrequency of translocation events by bindingmany of the free ssDNAs that would otherwisetranslocate (observed for the shorter bioti-nylated poly[dA]10) or else the capped complexproduces longer current blockages than other-wise observed (as with longer biotinylatedpoly[dA]50) [47]. Chandler et al. further charac-terized this biosensor system by examiningnonspecific adsorption of capped complexes tothe lipid bilayer, using simultaneous opticaldetection [48].
This sensing method is demonstrated for twoprobeanalyte complexes: biotinavidin andbromodeoxyuridineantibromodeoxyuridinepolyclonal antibody, although the authors claimit should be capable of detecting any analytethat can be coupled to, or substituted for, theabove complexes. Also, because different DNA
Figure 5. Classification of RNA diblock copolymer translocation events.
(A) Classification system used by Butler et al. for analysis of RNA diblock copolymer translocation events. (B) Example fit for a poly(C)50(A)25 event. Adapted with permission from [39]. 2006, Biophysical Society.
DataFitOpen
State
MidState
HiState
LoState
NoM
idM
id
NoStep HighLow LowHigh
High HighLow Low
Mid Mid Mid
High HighLow Low
BA140120100806040200
-200 200 400 600 800 1000
Time (s)
Curr
ent (
pA)
DataFitOpen
state
Midstate
High state
Low state465www.futuremedicine.com
encapsulation technique could prove important sequences give different event characteristics (as
-
REVIEW Healy
466discussed earlier in the Research with biologi-cal pores section), it is possible to detect multi-ple analytes simultaneously, which wasdemonstrated for these two analytes.
Nakane et al. used avidin-capped bioti-nylated ssDNAs in a similar way to create asequence-specific DNA sensor [49]. As shown inFigure 6, they used a long ssDNA that protrudesbeyond the end of the pore, with a specificsequence in this protruding region. Comple-mentary ssDNAs, if present, hybridize andthus prevent the complex from escaping thepore. Applying an opposite voltage forces thecapped complex back out of the pore, unbind-ing the hybridized ssDNA in the process. Thetime required for this unbinding measures theunbinding force required, and associated varia-tions in the statistical distribution of unbind-ing times are sensitive enough to detect singlebase-pair mismatches. This approach is termedforce spectroscopy.
Tropini and Marziali describe a clever exten-sion of this scheme using hundreds of -hemo-lysin pores incorporated in a single bilayer,enabling the same unbinding measurement tobe repeated hundreds of times in parallel [50].This is feasible because the exponential-likeensemble current signals (i.e., the sum of cur-
rents owing to each individual avidinDNAcomplex interacting with each individual pore)directly give a statistical description of theunbinding time. Compared with the originaltechnique, equivalent results can be achieved farfaster, with just a few of these parallel measure-ments replacing hundreds of sequential single-molecule unbinding events. The authors pro-pose applying this technique for genotyping andsingle nucleotide polymorphism detection byreplacing -hemolysin with synthetic pores fordurability and further parallelizing the systeminto an array, each element of which wouldmatch a specific DNA sequence, much like aDNA microarray.
DNA unzippingA force spectroscopy approach was also used bySauer-Budge et al. to study and model the unzip-ping kinetics of similar hybridized DNA seg-ments [51]. They omitted the avidinbiotin cap,simply placing the already hybridized DNA onone side of the pore. Based on analysis of thepore-blockage durations, taken to represent theunzipping time, the authors estimated the energybarriers to unzipping and also calculated theeffective charge per nucleotide while in the poreto be approximately 0.1 elementary electron
Figure 6. DNA sensor concept.
Method as used by Nakane et al. The plot shows applied voltage (red) and recorded current (blue) versus time. A biotinavidin-capped ssDNA is first driven into the pore, recognized by a drop in current. The presence of a hybridized strand is indicated by a lower-than-expected current when the voltage is reversed. This hybridized segment is unbound after some time owing to the applied force and the current increases. Single base-pair mismatches can be detected as statistical changes in the distribution of toff. Adapted with permission from [49]. 2004, Biophysical Society.
0.05 0.10 0.15 0.20 0.25
-100
0
100
200
Vrev
V (m
V), I
(pA)
0.30t (s)
toffNanomedicine (2007) 2(4) future science groupfuture science group
-
Nanopore-based single-molecule DNA analysis REVIEW
future science groupfuture science groupcharges (nucleotides have one negative elemen-tary charge each, but the effective charge can bereduced owing to the presence of counter-ions insolution, which are attracted to DNA moleculesand can be dragged along with them).
Math et al. examined the unzipping process inmore detail, this time forming the hybridized seg-ment in a single ssDNA molecule, by incorporat-ing two contiguous complementary segmentsthat folded and base-paired with each other,forming a hairpin structure [52,53]. They used areal-time feedback control system, capable ofautomatically detecting polynucleotide entry intothe pore, and then varied the applied voltage inresponse. This system was developed previouslyby Bates et al. and used to study the escapedynamics of linear ssDNA from the pore [54].Math et al. exploited this voltage control tomaintain rapid capture of molecules but, uponcapture, changed the voltage as desired to exploreunzipping at a variety of forces, including time-varying forces typically linear ramps. Resultsshowed different regimes for high and low ramprates, which their analysis and modeling deter-mined to be due to rezipping at lower forces. Thatis, at lower forces, the hairpins unzip and rezipmany times as a result of thermal fluctuations,however, higher forces prevent this rezipping.
The authors also used this feedback-control-led method to analyze the aforementioned corre-lation between orientation of a translocatingpolynucleotide and the resulting two groups oftranslocation events [38]. Hornblower et al.applied the same system to investigate pro-teinDNA interactions, studying the dissocia-tion of ssDNAExonuclease I complexes [55].Exonuclease I is too large to fit through and soholds the ssDNA in the pore until it dissociatesowing to the applied force. An independentmodel is presented describing the results, which,together with experimental data, gave an associa-tion rate constant consistent with previous studies(digestion of ssDNA by Exonuclease I was inhib-ited during these measurements, either by ssDNAphosphorylation or the absence of Mg2+). Theseresults are also important in the context of DNAanalysis because proteinDNA interactions pro-vide a way to slow down and regulate DNA trans-location velocity, enabling more information to begained from each translocation event.
Molecular switchesThe biotinavidin-capped sensor methodology isextended by Snchez-Quesada et al. using a
PEG end with biotinavidin and a DNA hairpinat the other [56]. This structure, consisting of acapped rod threading an aperture or ring, istermed a rotaxane and it forms a molecular switchor two-state detector because the ssDNA and PEGcause different levels of current blockage in thepore. The authors suggest that this approach couldbe useful for nanopore-based DNA analysisbecause it would enable the same molecule to bescanned many times, thus averaging outbackground noise.
Perhaps while carrying out these rotaxane exper-iments, some of the authors, with Ashkenasy,made the surprising discovery that, for a DNAhairpin with a single-stranded overhang threadingthe pore, the current blockage level was sensitive toa very specific position within the pore [57]. Thisposition corresponds to the 20th nucleotide fromthe start of the single-stranded section of thethreaded molecule and is predicted to be near theopening on the barrel side of the pore (Figure 2).
Single base-pair resolutionThis concept of using hairpin structures to pre-vent ssDNA from passing freely through the pore,thus enabling it to be observed for longer, was pio-neered by the AkesonDeamer group at the Uni-versity of California, Santa Cruz (CA, USA).Vercoutere et al. observed that blockade-currentsignals generally had a specific pattern (Figure 7), inwhich the current initially falls to an intermediatelevel, remains there for some time (referred to asthe shoulder region of the event) and then dropsbriefly to a lower level before returning sharply tothe initial value [58]. It was predicted that thesestages correspond to the hairpin first entering thewider vestibule of the pore, remaining there for atime until random thermal fluctuations happen tohave the right value combined with the electricfield-induced force to unzip it, and then rapidlytranslocating through the pore.
The energy required to unzip a hairpinincreases with its length and thus it wasexpected, and confirmed, that the durations ofthe shoulder regions should also increase withhairpin length because larger and thus statisti-cally less frequent thermal fluctuations would berequired. Also, the degree of blockage increaseswith hairpin length, consistent with the largerhairpins occupying more and more of the vesti-bule, thus displacing ions. These shoulder dura-tions and blockage depths exhibited highsensitivity to hairpin length, readily enabling sin-gle base-pair length differences to be identified467www.futuremedicine.com
ssDNAPEG diblock copolymer, capped at the between event classes.
-
REVIEW Healy
468An automated system was also developedthat gave the ability to characterize individualevents true single-molecule analysis using aseries of support vector machines (SVMs). Asdescribed in Box 2, SVMs enable automatedclassification of events or items that can bedescribed uniquely by a set of parameters. Thissystem was introduced by Vercoutere et al. [58].Briefly, events greater than a minimum dura-tion and within a certain blockade depth rangewere extracted from raw data by a simple algo-rithm, then each classified automatically for 36different parameters, such as shoulder regionduration and several statistics of blockagedepth. The values of these parameters form36-element vectors representative of each event,which are input to the SVMs.
This automated analysis enabled the identifi-cation of single base-pair hairpin length differ-ences, single nucleotide length differences inthe hairpin loop and single base-pair mis-matches within the hairpin stem, all with veryhigh confidence (
-
Nanopore-based single-molecule DNA analysis REVIEW
future science groupfuture science group
Figure 8. 2D suppor
In this 2D example, paramparameter choices result class, for which a maximadata points are then classthey fall, with a confiden
computer (quantum computers are a potentialcandidate [70,71]). Owing to this difficulty, todaysprotein-engineering techniques typically involvemaking very small changes to existing well-char-acterized proteins and then experimentallydetermining the structural modifications.
Howorka et al. modified standard -hemo-lysin to include a single cysteine amino acid nearits cis opening by modifying the DNA sequencethat specifies the -hemolysin amino acid
sequence and then borrowing natures machineryto produce the modified protein [72]. Sub-sequently, ssDNA molecules were modified witha sulfur-bearing group at the 5 end and cova-lently bonded to this cysteine according tostandard techniques. Because only one cysteine ispresent, only one ssDNA will be bonded.
This engineered -hemolysin was then usedfor analyzing linear ssDNA molecules, asdescribed previously for standard -hemolysin.Owing to base-pairing with the tethered ssDNA,complementary sequences were slowed whilepassing through the pore, resulting in differentcurrent-blockade characteristics. This enabled:
The identification of specific sequences, forexample, a sequence specific to a drug-resistance-conferring mutation of HIV
Differentiation between single base-pairmismatches
Determination of the position of thesemismatches
For the HIV mutation, individual moleculeswere identified with an accuracy of 93% in alimited test involving 75 detections.
This work also enabled the study of DNA-hybridization kinetics [73]. Results were reported tobe similar to macroscopic studies on hybridizationin free solution, suggesting that confinement in thenanopore does not significantly affect this behav-ior, although a slight voltage dependence wasobserved. This suggestion was reinforced by a laterstudy using longer ssDNAs with additional over-hanging bases after the complementary segment[74]. When the complementary sequences arehybridized, these overhanging regions will threadfurther into the pore, thus modifying the current
Box 2. Support vector machines.
Somewhat ambiguously named, a support vector machines (SVMs) separates n-dimensional vectors (i.e., points in n-dimensional space) into one of two classes. It is helpful to look at a 2D version (Figure 8) because the geometrical methods involved are applicable in any n-dimensional space.Each class is defined by a series of 2D parameter vectors known to belong to that class, that is, a set of points in 2D space. Parameters need to characterize classes uniquely, so that related points form clusters this is why a large number of parameters are used in practice.The key to the SVM is the calculation of an optimal separation line (plane/hyperplane at higher dimensions), maximally and equally distant from each cluster. Classification of an unknown data point is then simply a matter of testing on which side of the separation line it resides, with a confidence level given by its distance to the line. The separation line is typically assigned a thickness, corresponding to the minimum acceptable confidence level. The SVM name derives from the parameter vectors closest to the separating line, called the support vectors.As a standard SVM only makes a yes/no decision, several are typically cascaded when differentiation between more than two classes is required. More complex designs also exist; for example, using hyperspheres to define each class of events [120].
t vector machine classification.
eter vectors correspond to 2D points. Suitable in spatial clusters of points corresponding to each lly and equally separating line is calculated. Unknown ified based on the side of the separating line on which ce measure given by their distance from it.
Optimalseparationline469www.futuremedicine.com
blockade signal. Because the overhang length is
-
REVIEW Healy
470known, this modified blockade signal can be corre-lated to a particular depth within the pore and,thus, with a series of different overhangs, theauthors were able to probe the structure of the poreand also the electric field distribution within it.They found, as would be expected from the knowncrystal structure of -hemolysin [31], that the fieldstrength is lower in the wider vestibule region, con-sistent with the slight voltage dependence observedearlier. Electric-field distribution within the porehas also been studied recently by moleculardynamics simulation [75].
Another engineered -hemolysin variantwith a ring of seven positive charges within thepore barrel was used by Astier et al. in anattempt to detect translocation of individualnucleoside monophosphates (i.e., single A, G,T or C molecules) [76]. This is an interestingapplication because it could be a potentialdetection technique for an exonucleasesequencing method [77], in which a DNA mole-cule is sequenced by repeatedly removing anddetecting its terminal base.
The engineered pore alone did not show suffi-cient sensitivity, so the authors used the amino-cyclodextrin am7CD as a molecular adapter.am7CD is a ring-shaped molecule that fits intothe -hemolysin barrel, aligning with the posi-tively charged ring and exposing its own ring ofpositively charged amino groups. This adapternarrowed the channel and, together with its pos-itive charges, enabled the detection of individualnucleoside monophosphates. Reported accura-cies were high: 98, 93, 97 and 96% for G, T, Aand C, respectively. This report was for deoxyri-bonucleoside monophosphates (i.e., DNA) butsimilar results are claimed for RNA.
Synthetic poresDespite -hemolysins precise structure, relativeease of use and potential for protein engineering,
approximately 36 h, owing to the fragility of thelipid bilayer [40], which is not sufficient for a prac-tical analysis tool. Its biological nature also limitstolerance of chemical extremes, such as high orlow pH, which are likely to be required to elimi-nate secondary structure in real-world DNA sam-ples. Similarly, to avoid background fluctuationsin the -hemolysin pore current, experimentsmust be carried out within a specific range of saltconcentrations, typically 1 M or higher. As aresult, -hemolysin cannot be used to studyDNA or other biomolecules in physiologicallyrelevant conditions. These disadvantages havedriven the development of synthetic nanopores(recent developments with supported [78] andencapsulated [46,79] bilayers have begun to addresssome of the disadvantages).
The first report of DNA detection with a syn-thetic nanopore was by Li et al. in 2001 a briefmention of blockade events observed with 500-bpdsDNA and a silicon nitride pore [80]. Much hasbeen accomplished since then with syntheticpores but, as can be seen from this review, thequantity of research and the abilities demon-strated have yet to rival -hemolysin. This com-parison is somewhat hampered by the lack ofoverlap between published studies. This is cer-tainly partly owing to the difficulty with currenttechniques of repeatably fabricating syntheticpores of equivalent size, although another contri-bution seems to be researchers natural preferencefor new and unique experiments.
Comparisons among synthetic pore experi-ments are also more difficult. Each manufactur-ing process produces pores with differentcharacteristics, and even different pore sizesfrom the same process can give different results.Nanopore-based DNA analysis has been carriedout with synthetic pores fabricated in siliconnitride, silicon dioxide and polymer materials,such as polyimide and polycarbonate. Also,
Box 3. Hidden Markov models.
Hidden Markov models (HMMs) statistically model random processes whose future states depend only on the current state and a finite number of previous states, often referred to as Markov processes. Brownian motion is an example of a Markov process, as are the hairpin event signals analyzed by Winters-Hilt et al. [59]. Models consist of a set of hidden states with associated sets of emission and transition probabilities. While in a given state, the probability of a particular output is set by the emission probability, whereas the probability of transition from that state to each of the other states is the transition probability. One common method to construct a HMM is expectation maximization. Starting from a generic set of probability values, the model is built, or trained, by adjusting these probabilities in a step-by-step fashion so that they describe the signal being modeled more and more closely. These probability sets provide a detailed and inherently statistical description of the signals analyzed and thus make efficient parameters for support vector machine classification.Nanomedicine (2007) 2(4) future science groupfuture science group
it has disadvantages. Its lifetime is typically proof-of-concept DNA-translocation results for
-
Nanopore-based single-molecule DNA analysis REVIEW
future science groupfuture science groupsome further materials and manufacturingmethods have been reported recently [8183].Nanopore manufacturing processes will be cov-ered in a separate review [84]. Silicon nitride hasbeen the material used most frequently to date,so the material type will only be specified infurther discussions if it differs.
After the initial report, 2 years passed beforethe next, again by Li et al. [85]. Some of thisdelay might be explained by a brief commentrelegated to small print on the last page of thepaper: Pre-soaking the chip in isopropanol wasfound to aid wetting the pore. Many othershave since reported similar difficulties wettingpores manufactured by related methods [8688].
Given the difficulty of producing smaller syn-thetic pores, practically all work performed todate has examined dsDNA. Notable exceptionsare Heng et al. [89], who somewhat unusuallyhave only reported DNA analysis results withpores of 3 nm diameter or smaller (as theyreport having produced pores up to 10 nm indiameter [90]). Similar to -hemolysin, with
ssDNA and not dsDNA. Also, Fologea et al.studied events at increasing pH values andobserved denaturation of dsDNA into singlestrands [91]. This latter work is important in thecontext of practical applications of nanopore-based DNA analysis because they will probablyrequire denaturation to remove secondary struc-ture that will be encountered in genomic DNAand would otherwise interfere with the translo-cation process. More recently, Harrell et al. alsoexamined ssDNA (7250 nt in length) withmuch larger (40 nm diameter) polycarbonatepores [92]. As the authors state, these experi-ments were perhaps closer to traditional resis-tive-pulse Coulter counting because the DNAmolecules could pass through the pore in rela-tively random configurations owing to its largesize. However, they were still sufficient to clearlydistinguish the ssDNA from a similar length ofplasmid DNA.
Researchers have also provided confirmationthat DNA does indeed translocate through thepore. Chen et al. observed individual fluores-
Figure 9. Physical interpretation of current blockade fluctuations.
Data as observed by Vercoutere et al. (A) The IL conductance state. One of the terminal nucleotides interacts with amino acids in the vestibule wall. (B) The UL conductance level, where the terminus has desorbed from the pore wall and is free to explore orientations within the vestibule that enable increased current through the pore. (C) The LL conductance level. In this state, one or both nucleotides of the terminal base-pair interact with amino acids, such that one or both nucleotides are poised directly over the pore-limiting aperture. When one or more base pairs at the duplex terminus fray from this position (D), one of the two strands extends from its helical conformation and penetrates the limiting aperture of the pore (E). The duration of the current versus time recording shown was not specified but it is in the range of 50300 ms. Adapted from [61] with permission of Oxford University Press.
B CA D E
IL UL LL F S
120
pA
0
ULILLL
S S471www.futuremedicine.com
small pores, they only detect translocations of cently tagged -phage DNA molecules
-
REVIEW Healy
472
Table 1. Order-of-msynthetic nanopore
First author Diam
Meller (-hemolysin)Chang
Healy
Aksimentiev**
Li
Mara
Storm
Data are calculated from t-hemolysin data are showdsDNA. Pores were assumeto two significant figures. unless specified. *Approximate effective leUnits are nt s-1 because t0.1 M KCl.Unpublished data, Healy e#Length over which 85% odrop is nonlinear however**Molecular dynamics sim
e: Electrophoretic mobilitapproaching the pore and then disappearing [93],whereas Heng et al. carried out PCR analysis,detecting the used DNA successfully at thetransmitted side of the pore [89,94]. However,unlike results with -hemolysin, the quantity ofmolecules estimated by quantitative PCRexceeded the number of events observed [95].Their explanation is that many events are too fastto be observed within the 100-kHz amplifierbandwidth because translocations through thesepores are predicted to be approximately 40-timesfaster than with -hemolysin [94].
Length measurementMost work to date has focused on dsDNA mol-ecule length measurement. Li et al. compared 3-and 10-kb fragments using a 10-nm pore andshowed essentially nonoverlapping peaks in theevent duration histogram [85]. Mara et al. exam-ined shorter lengths 286, 974 and 4126 bp(4 nm polyimide pore) and found correspond-ing histogram peaks [96]. However, these weresignificantly overlapping and hence the abilityto identify single or small numbers of moleculesis limited (significantly overlapping peaks willbe arbitrarily defined here as those that intersecttheir neighbors above the half-maximum valueof either peak). Storm et al. looked at 6.6-, 9.4-,
11.5-, 27.5-, 48- and 96-kb fragments (10-nmsilicon dioxide pore), with only the 6.6- and9.4-kb and 9.4- and 11.5-kb pairs of peaks sig-nificantly overlapping [97]. Heng et al. examined100-, 600- and 1500-bp lengths and also com-pared 50-bp dsDNA with 50 nt ssDNA. Eachevent class did show identifiably different statis-tical behavior, but peaks of shorter lengths (andthe ssDNA) were essentially completely over-lapped by those of longer lengths. Recently, Fol-ogea et al. refined the length measurementprocess by taking into account both event depthand duration [98]. See the Folded DNA sectionlater for a discussion.
The electrophoretic mobility of translocatingDNA molecules (i.e., the velocity divided by theelectric field strength) appears to vary widelybetween different pore types. Table 1 presentscrude estimates of electrophoretic mobility,based on translocation durations collected fromthe literature. As discussed in the Research withbiological pores section, it has been reported for-hemolysin that molecules shorter than thelength of the pore translocate faster, that is, theelectrophoretic mobility is higher [43]. However,none of the translocation durations used inthese calculations corresponded to moleculesshorter than the pore length. Given this wide
agnitude estimates of electrophoretic mobility for DNA translocating through various s.
eter(nm)
Pore length(nm)
Appliedvoltage (V)
Average electric fieldstrength (kV cm1)
e vt Ref.
1.5 7.5* 0.12 160 12 0.560 [35]
10.0 36.0 0.20 56 3 0.030 [105]
12.0 120.0# 1.70 140 23 0.066
2.4 5.2 1.40 2700 510 34.000 [121]
10.0 7.5 0.12 160 570 27.000 [85]
4.0 120.0# 0.12 10 920 2.700 [96]
10.0 20.0 0.12 60 2200 39.000 [88]
ranslocation times reported in the literature and simplistic estimates of the electric field strength for each case. n for comparison but the mobility listed cannot be compared directly with other values because it relates to ssDNA not d cylindrical unless otherwise specified, with the electric field strength given as E V/L. All values shown are truncated If multiple values or ranges were given in the references cited, the values here are averages. All results are for 1 M KCl
ngth, given that the vestibule is wider and thus field strength will be lower there.
his is ssDNA.
t al. (polyimide pore, similar to [96], 1215 bp dsDNA, mean translocation time for 529 observed events was 1.29 ms).
f the voltage drop occurs in these conical polyimide pores (see supporting information accompanying [15]). This voltage , so, in reality, the peak field (at the cone-tip) will be higher than the average value given here.ulation.
y (nm2 V-1 s-1); vt: Estimated translocation velocity at 120 mV (bp s-1).Nanomedicine (2007) 2(4) future science groupfuture science group
-
Nanopore-based single-molecule DNA analysis REVIEW
future science groupfuture science groupvariation, it is clear that pore characteristicsmust influence DNA behavior significantly.Values cannot be compared with those for DNAin free solution (e.g., [99]) because, in this situa-tion, molecules have a coiled conformation andessentially move as globular particles withequivalent charge.
As noted previously, it has been reported thatthe effective charge on DNA in a nanopore issignificantly reduced owing to the presence of acounter-ion cloud that moves with it [51]. Dis-ruption of these counter-ions (e.g., by acharged surface or steric effects in a sufficientlysmall pore) will change the force experienced bythe DNA and thus its mobility. With referenceto Table 1, Mara et al. (1.5 e/nm2, Z Siwy, Pers. Com-mun.), Li et al. (see [100], charge not quantified)and Chang et al. (45 e/nm2) have reportedthat the pores used in their experiments havecharged surfaces.
Another possibility is that significant electro-osmotic flow is likely to occur is these channels,also a consequence of surface charge (near acharged surface, anion and cation concentrationsare distorted opposite to the surface charge, sothat when an electric field is applied, the fric-tional forces exerted on the surrounding solutionby each polarity of ion are similarly unbalanced,resulting in a net flow). Owing to the materialsused and at the pH values in question, surfacecharges will be negative. This means that theelectro-osmotic flow, if present, would betowards the cathode, thus opposing DNA trans-location. These possibilities of reduced trans-location velocity due to hydrodynamic andelectrostatic drag forces, from the electro-osmoticflow and underlying cation flow, respectively,were also noticed by Chang et al. [86].
Storm et al. report another complication,finding that electrophoretic mobility decreaseswith DNA length according to a power law,e L/t L(1-), where e is the electrophoreticmobility, L is the DNA length, t is the eventduration and = 1.27 is the power law constant[97]. They describe a model that agrees quantita-tively with this power law, on the basis that, forlong polymers in short pores, hydrodynamicdrag on the coil of polymer yet to translocatedominates over friction on the section of poly-mer within the pore. As a result, this drag forcedictates translocation time, and larger polymerswill encounter larger drag forces, thus reducingmobility. An equivalent study with other poretypes would be very valuable to determine
For practical applications, the translocationvelocity is more relevant because this will limit theresolution of any analysis system. Table 1 also liststhe equivalent velocities, scaled if necessary to120 mV applied voltage. Reducing voltage willobviously reduce velocity but thermal fluctuationsare unchanged and thus the signal:noise ratio isreduced. This is discussed further below.
Folded DNAWhile analyzing translocation events from a10-nm pore, Li et al. noticed some with aninteresting multilevel structure, in which thecurrent is always one of two discrete levels, thedepth of the lower being twice that of the upper[85]. This led to the theory that dsDNAs can befolded and can pass through the pore in a dou-bled-up configuration. This is conceivable,given that a 10-nm pore could probably containseveral DNA helices because each is approxi-mately 2 nm in diameter. Further evidencecame from the fact that the areas (see integralequation below) of all single- and multi-levelevents were similar and from a histogram of cur-rent levels, I(t) I0, for all events (i.e., the rawdata points), which showed clear peaks, with thelevel of the second twice that of the first.
Chen et al. [93] and Storm et al. [88] (silicondioxide pores) followed this with experimentsusing the longer -phage DNA, which elegantlyhighlighted this folding behavior (Figure 10).They developed automated software to unwrapthe multilevel events and, as expected, foundthat the translocation times of these were statisti-cally equivalent to those of single-level events.They also further confirmed the folding theoryby observing that, with circular DNA, events ofthe smallest depth level are eliminated.
In these reports, the unfolded state accountedfor more than 50% of recorded events in eachcase and the frequencies of the folded-in-n statesdecreased exponentially with n. This foldingbehavior highlights the forces being exerted,given that the persistence length of a free dsDNAmolecule (essentially the minimum length overwhich a bend of one radian will occur) at roomtemperature and the ionic strengths used here(1 M KCl) is more than 45 nm [101,102].
Fologea et al. have exploited this foldingbehavior to simultaneously determine lengthand conformation of DNA molecules to giveperhaps the most practical realization to date of ananopore-based laboratory DNA analysis tool[98]. They determined conformation (e.g., single-473www.futuremedicine.com
whether this is a universal effect. stranded, double-stranded, folded double-
-
REVIEW Healy
474
Figure 10. Translocacorresponding to fo
Data as observed by Storpossible configurations scomplex forms. (E) Histoshows clear quantizationAdapted with permission
-7.2-7.1-7.0-6.9
0
I (nA)
I (nA)
I (nA)
I (nA)
-7.2-7.1-7.0-6.9
0-6.8
-7.2-7.1-7.0-6.9
0-6.8
-7.2-7.1-7.0-6.9
0
-6.8-6.7-6.6-6.5
B
A
E
1
10
100
1000
10,000
100,000
1,000,000
Coun
ts
-100 0
0
1stranded or supercoiled) based on event depthand length based on event area, which they termevent charge deficit:
where I0 is the open pore current, I(t) is the cur-rent level during the event, tx is the start time ofthe event and T is its duration. Analysis of astandard DNA ladder mixture is used to cali-brate event depth and area. Using this techniquein blind tests, they determined length and con-formation of linear and relaxed-circular ssDNAand dsDNA samples successfully. Lengths stud-ied were in the range of 2.610 kbp and all weredetermined successfully within confidence lim-its that ranged from 0.2 (for 2.6 kbp dsDNA)to 3.8 kb/kbp for (10 kbp dsDNA). Theseaccuracies are still worse than current electro-phoresis techniques, although the run time(30 min) is significantly faster.
Event polarityGiven a simple macroscopic analogy to Coultercounter operation the flow through an aper-ture is reduced when an object partially blocksthat aperture results presented by Chang et al.are quite surprising, where transient increases inmeasured current were observed, supported byPCR analysis showing that translocations wereindeed taking place [86]. However, the surprisehere is because of failure of the analogy. In fact,as was noted by Coulter, the quantity beingsensed is conductance and, thus, the fluidmedium and the particles must have differentconductivities [302]. Thus, it follows that parti-cles with higher effective conductivities willcause increases in current.
Subsequently, Fan et al. (albeit in muchlonger 10-m nanotubes) [103], Smeets et al.[104] and Chang et al. [105] all observed the grad-ual transition from current reductions to cur-rent increases as the solution conductivity isreduced by reducing the KCl concentration.
At the molecular level of translocating DNA,these current increases are explained as the con-tribution of counter-ions moving with the DNAmolecule. Current carried by DNA moleculesthemselves is negligible because their electro-phoretic mobilities are far lower than simpleions, such as K+ and Cl [105]. Chang et al. pro-vide the most theoretically based model to date,which also predicts that this counter-ion currentwill saturate with increasing electric field [105].This is owing to polarization of the counter-ion
tion events and current histogram lded -phage DNA.
m et al. (A) Example events owing to the range of hown diagrammatically in (BD) and some more gram based on all data points for 1598 events that into multiples of the smallest blockage class. from [88]. 2005 by the American Physical Society.
5 10 15 20 25
5 10 15 20 25
5 10 15 20 25
5 10 15 20 25Time (ms)
C D
100 200 300 400 500 600 700 800 900
1
2
3
45
I - Ibaseline (pA)
2 21
1
2
21
2432 2132
I00
T I tx t+( )dtNanomedicine (2007) 2(4) future science groupfuture science group
-
Nanopore-based single-molecule DNA analysis REVIEW
future science groupfuture science groupdistribution on the DNA molecule, ultimatelyleading to depletion of counter-ions at one end,which they describe as similar to channel pinch-off in a field effect transistor. This saturationmeans that, with increasing voltage, the polarityof events will transition from current increasesback to current decreases, which they haveobserved experimentally.
All of the measurements described in thissection used silicon dioxide pores.
Trapped DNAAs with -hemolysin, the ability to restrict themotion of DNA in synthetic pores is valuable inenabling molecules to be observed for longerdurations and thus allowing more information tobe gathered. Fologea et al. explore the effects ofvarying experimental conditions increasingsolution viscosity with glycerol, reducing tempera-ture and decreasing voltage which cumulativelyenabled a factor of ten reduction in translocationvelocity [106]. However, these variations will simi-larly reduce ion mobilities and thus current.Nanopore current noise, according to a shot noiseassumption [28], will increase with the square rootof current and thus the signal:noise ratio isreduced at these lower currents. Other noise com-ponents might also be unaffected by these experi-mental changes (e.g., although thermalfluctuations would be expected to reduce withincreasing viscosity or decreasing temperature,they will be unaffected by voltage). This approachmight still be useful, however, if additional infor-mation gained by the longer observation timeoutweighs the reduction in signal:noise ratio.
Keyser et al. demonstrate a method to controlDNA position mechanically (Figure 11) [107,108].They attach dsDNA molecules to a polystyrenebead, then position it close to a 10-nm silicondioxide nanopore [109] using an optical trap.With an applied voltage, a DNA moleculeattached to the bead is drawn into the pore, as instandard translocation experiments, and thenheld stretched, the force due to the electric fieldin the pore balanced by the optical trap. Thistrap force can be measured based on the distanceof the bead from the optical focus, and thus ena-bled the DNA effective charge in this situationto be calculated as 0.5 elementary charges perbase pair. This value was also constant over theKCl concentration range of 0.021 M, which, asstated by the authors, is in disagreement withexisting theory that instead expects force todecrease with increasing concentration. An
Another approach to restrict DNA motion ischemical modification of the nanopore walls.Iqbal et al. have attached DNA hairpin loopscovalently to the silicon dioxide walls of theirnanopores by amino silane chemistry and haveexamined their influence on the ion current sig-nals in the presence of complementary and mis-matched ssDNA [110]. This is based on previouswork by Kohli et al. in which the bulk transportof ssDNA was investigated through membranescontaining many gold nanotubes modified withsimilar hairpin loops [111]. These investigationsshowed that the hairpin loops enhanced trans-port of complementary ssDNA selectively byacting as transporters, enabling ssDNA mole-cules to pass through the nanotubes by jumpingfrom one hairpin loop to the next (i.e., dehybrid-izing from one and hybridizing to the next).With suitably designed hairpin loops, this trans-port can be selective even for a single-base differ-ence because hybridization is blocked for themismatched DNA.
ConclusionIt is clear that DNA is of key importance in bothbiological research and medical diagnosis owingto its fundamental role in living organisms.Although DNA analysis technology hasadvanced significantly in recent decades, spurredespecially by the Human Genome Project, it stillonly enables us to scratch the surface of a com-plete understanding of the role of DNA in life.
New single-molecule techniques aim to helpus delve deeper, by way of increased speed, moreeconomy and new capabilities. Nanopore-basedDNA analysis is one such technique and hasbeen described in detail here. Based on the Coul-ter counter concept, it attempts to analyze DNAmolecules based on the changes in ion fluxthrough a nanometer-diameter pore caused by aDNA molecule traveling linearly through it.Since the first report in 1996 using the biologicalnanopore -hemolysin, much progress has beenmade. Researchers have succeeded in achievingsingle-base resolution and recognition of indi-vidual nucleotides in certain specific situations.Much has also been learned about the behaviorof ions and polymers on this minute scale.
More recently, the technique has also beendemonstrated using synthetic nanopores. Inter-est in these pores stems from their potential toenable customized structures and mass produc-tion. Also, their increased strength and durabil-ity give longer lifetimes and enable operation in475www.futuremedicine.com
explanation has yet to be determined. the harsher conditions that will be necessary to
-
REVIEW Healy
476optimize DNA behavior. Results with syntheticpores have yet to match the precision of thosewith -hemolysin, although they have alreadyenabled experiments impossible with biologicalpores, such as monitoring DNA denaturation aspH is increased.
Future perspectiveNanopore-based DNA analysis has the potentialto be revolutionary; however, several obstaclesremain towards a practical nanopore-based DNAanalyzer. A probable first application is to replaceelectrophoresis as the tool for DNA length meas-urement. It already has far superior speed but therequired single base-pair resolution has yet to beachieved over length ranges comparable with
electrophoresis, that is, 6001200 bp (by far themost common application of DNA length meas-urement is as part of the DNA sequencing proc-ess, which requires single base-pair resolution).
Achieving this will require a combination ofmore sensitive detection electronics and methodsto slow down or control the motion of DNAmolecules passing through the pore. Secondarystructure must also be eliminated, probably byoperating the system under denaturing condi-tions. Some initial progress has been madetowards these goals, as mentioned here.Advancements in nanopore technology will alsobe required. Pores for this system will need relia-ble and repeatable fabrication methods capableof volume production at practical costs. Current
Figure 11. Double-stranded DNA attached to an optically trapped bead being threaded through a nanopore.
As demonstrated by Keyser et al. [106,107]. The plots show ionic current and bead position for a single threading event. Bead displacement from the optical trap focus gives the force exerted. Experimental conditions were 1 M KCl and 33 mV applied voltage, and signals were filtered at 10 kHz. Adapted with permission from Macmillan Publishers Ltd; Nature Physics [107], 2006.
3.50
3.55
0 10 20 30 40
Time (ms)
Time (ms)
Curr
ent (
nA)
DNA entersnanopore
t
0
0.1
0.2
0 10 20 30 40
Z (
m)
ZNanomedicine (2007) 2(4) future science groupfuture science group
-
Nanopore-based single-molecule DNA analysis REVIEW
future science groupfuture science group
Executive summary
Nanopore-based DNA
Nanopore-based DNAchambers of electrolyelectric current.
When molecules passon the degree and du
This technique promismeasurement, specifi
Nanopores
Initial work used a biostructure. Fortuitouslytrapped within or tran
-hemolysin continuetailored capabilities.
However, -hemolysinlipid bilayers in whichsalt concentration), w
As a result, several typanalysis experiments w
Experimental highligh
-hemolysin has provienabled DNA-based bnucleoside monophos
True single-molecule aclassifies each individunucleotide resolution
Synthetic pore experim0.3100.0 kbp, and mSynthetic pores have and observation of fomethods have yet to demonstrate sufficientrepeatability and all are time- and labor-intensiveprocesses, requiring expensive, large-scale equip-ment. These requirements for high-accuracy vol-ume production are being addressed and somepreliminary progress has been reported [112,202].
More ambitious applications, such as analyzingand even sequencing chromosomal DNA directfrom individual cells, bring further challengesbeyond those discussed already. First, similar to allsingle-molecule methods focusing on this area, anautomated system is needed to extract and isolateDNA molecules from individual cells and thendeliver them to the detector (i.e., in this case thenanopore). Much has been accomplished in thisdirection with microfluidics but the last micronproblem still requires attention; that is, preciselytransporting the molecule this last micron to ananometer-sized pore or detection volume. Chro-mosomal DNA, from higher organisms at least,also requires careful handling its long lengthmakes it significantly more fragile compared withshorter fragments.
Second, resolving structural details, such assequence and nucleotide modifications (e.g.,methylation), is perhaps the ultimate challengefor a nanopore-based analysis system. In addi-tion to requiring sufficient time resolution todetect single bases, current resolution must behigh enough to distinguish minute changesowing to the various nucleotide structures.Among other requisites necessary to achievethis will be integration of electrodes and detec-tion electronics in close proximity to the poreand precise manipulation of DNA molecules:extending them, holding them in position andmoving them with subnanometer accuracy.
To meet some of these challenges, alternativesensing methods might be used in addition to orin place of the ionic current measurements usedto date. For example, Timp and coworkers haveproposed and simulated a capacitive sensingtechnique in which the nanopore is formedthrough a capacitor membrane [113115]. Here,translocating DNA molecules give rise to charac-teristic electrical signals on the capacitor related
analysis concept
analysis involves using an applied voltage to drive DNA molecules through a narrow pore, which separates te solution. This voltage also drives a flow of electrolyte ions through the pore, measured as an
through the pore, they block the flow of ions and, thus, their structure and length can be determined based ration of the resulting current reductions.es to carry out a range of analyses, orders of magnitude faster than current methods, including length c sequence detection and even de novo sequencing.
logical nanopore, -hemolysin, which provides a predesigned, atomically precise and repeatable pore , the composition and structure of the inner pore walls result in many interactions with a DNA molecule slocating the pore.s to be used in many experiments, including genetically engineered forms, which provide
pores are unlikely to be used in practical applications, owing to their short lifetime caused by fragility of the they are incorporated and their sensitivity to extreme environmental conditions (e.g., temperature, pH and hich will probably be required to control DNA conformation.es of synthetic pore have been developed to provide improved durability and lifetime. A number of DNA ith these pores have now been reported.
ts & achievements to date
ded as yet unrivaled results with short single-stranded DNA and RNA molecules and DNA hairpins. It has also iosensing, and genetically engineered -hemolysin has been used to detect and identify individual phates.nalyses have been achieved for short DNA hairpins and related molecules; that is, automated analysis al molecule with high confidence. These analyses include determination of hairpin length with single-
and identification of hairpin terminal base pairs.ents have studied double-stranded DNA molecules with lengths in the range of approximately olecule populations with length differences down to approximately 50% have been clearly distinguished.
also enabled discrimination between single- and double-stranded DNA, observation of DNA denaturation lded and supercoiled DNA conformations.477www.futuremedicine.com
-
REVIEW Healy
478
acid-based electrical detection systems. Microb. Cell Fact. 5, 9 (2
4. Deamer DW, Akeson Mnucleic acids: prospects sequencing. Trends Biote(2000).
5. Deamer DW, Branton Dof nucleic acids by nanoChem. Res. 35, 817825
6. Nakane JJ, Akeson M, MNanopore sensors for nuJ. Phys. Condens. Matter(2003).
7. Rhee M, Burns MA: Natechnology: research trenapplications. Trends Biot(2006).
8. Dekker C: Solid-state naNanotechnol. 2, 20921
9. Wanunu M, Meller A: Sanalysis of nucleic acids interactions using nanopManual on Single Molecu(Eds). Cold Spring Harb(2007).
Bayley H: Detecting protein analytes that channel. Proc. Natl Acad. Sci. USA 93,
006).: Nanopores and for ultrarapid chnol. 18, 147151
: Characterization pore analysis. Acc. (2002).arziali A:
cleic acid analysis. 15, R1365R1393
nopore sequencing ds and
echnol. 24, 580586
nopores. Nat. 5 (2007).ingle molecule and DNA-protein ores. In: Laboratory les. Ha T, Selvin P or Press, NY, USA
modulate transmembrane movement of a polymer chain within a single protein pore. Nat. Biotechnol. 18, 10911095 (2000).
13. Sun L, Crooks RM: Single carbon nanotube membranes: a well-defined model for studying mass transport through nanoporous materials. J. Am. Chem. Soc. 122, 1234012345 (2000).
14. Howorka S, Nam J, Bayley H, Kahne D: Stochastic detection of monovalent and bivalent protein-ligand interactions. Agnew. Chem. Int. Ed. 43, 842846 (2004).
15. Heins EA, Siwy ZS, Baker LA, Martin CR: Detecting single porphyrin molecules in a conically shaped synthetic nanopore. Nano Lett. 5, 18241829 (2005).
16. Siwy Z, Trofin L, Kohli P, Baker LA, Trautmann C, Martin CR: Protein biosensors based on biofunctionalized conical gold nanotubes. J. Am. Chem. Soc. 127, 50005001 (2005).
17. Stefureac R, Long YT, Kraatz HB, Howard P, Lee JS: Transport of -helical peptides through -hemolysin and aerolysin pores. Biochemistry 45, 91729179 (2006).
1377013773 (1996).20. DeBlois RW, Bean CP: Counting and sizing of
submicron particles by resistive pulse technique. Rev. Sci. Instrum. 41, 909916 (1970).
21. DeBlois RW, Bean CP, Wesley RKA: Electrokinetic measurements with submicron particles and pores by resistive pulse technique. J. Colloid Interface Sci. 61, 323335 (1977).
22. Hille B: Ion Channels of Excitable Membranes (3rd Edition). Sinauer, Sunderland, MA, USA (2001).
23. Mueller P, Rudin DO, Tien HT, Wescott WC: Reconstitution of cell membrane structure in vitro and its transformation into an excitable system. Nature 194, 979980 (1962).
24. Bean RC, Shepherd WC, Chan H, Eichner J: Discrete conductance fluctuations in lipid bilayer protein membranes. J. Gen. Physiol. 53, 741757 (1969).
25. Hladky SB, Haydon DA: Discreteness of conductance change in bimolecular lipid membranes in presence of certain antibiotics. Nature 225, 451453 (1970).to their charge, structure and displacement ofions and solvent molecules. Branton,Golovchenko and coworkers plan to study tun-neling currents between a conducting nanotubeand a conductive layer on the pore surface. As aproof of concept, they have recently probed a sil-icon nitride nanopore [80] using a 105-nm longcarbon nanotube grown on an atomic forcemicroscope tip, while simultaneously measuringthe current blockage [116]. Tunneling current isexpected to be influenced by the presence ofmolecules, such as ssDNA, adsorbed on thenanotube surface. Electron tunneling throughfreely translocating DNA molecules has alsobeen simulated between pairs of transverse elec-trodes surrounding the pore [117,118], and devicesintended to experimentally explore this sensingapproach have been fabricated recently [119].
Obviously, at this early stage, it is not guaran-teed that nanopore-based DNA analysis willachieve its revolutionary goals. It is even possible
that it might be eclipsed by an entirely newapproach, perhaps stemming from the ever-more-precise nanoscale fabrication techniquesoriginating from the semiconductor industrysdrive for ever-decreasing feature sizes. However,based on the progress since the inception of thisnanopore field in the mid-1990s and the rapidgrowth of its membership, future prospects fornanopore-based DNA analysis are high.
AcknowledgementsI thank B Schiedt, Z Siwy, AP Morrison, K McCarthy andS Winters-Hilt for insightful discussions and careful readingof the manuscript and D Deamer for his first-hand accounton the origins of nanopore-based DNA analysis.
Financial disclosureThe authors have no relevant financial interests, includingemployment, consultancies, honoraria, stock ownership oroptions, expert testimony, grants or patents received orpending, or royalties related to this manuscript.
Bibliography1. Marziali A, Akeson M: New DNA
sequencing methods. Annu. Rev. Biomed. Eng. 3, 195223 (2001).
2. Metzker ML: Emerging technologies in DNA sequencing. Genome Res. 15, 17671776 (2005).
3. Gabig-Ciminska M: Developing nucleic
10. Meller A: Dynamics of polynucleotide transport through nanometre-scale pores. J. Phys. Condens. Matter 15, R581R607 (2003).
11. Bezrukov SM, Vodyanoy I, Parsegian VA: Counting polymers moving through a single-ion channel. Nature 370, 279281 (1994).
12. Movileanu L, Howorka S, Braha O,
18. Krasilnikov OV, Rodrigues CG, Bezrukov SM: Single polymer molecules in a protein nanopore in the limit of a strong polymerpore attraction. Phys. Rev. Lett. 97, 018301 (2006).
19. Kasianowicz JJ, Brandin E, Branton D, Deamer DW: Characterization of individual polynucleotide molecules using a membrane Nanomedicine (2007) 2(4) future science groupfuture science group
-
Nanopore-based single-molecule DNA analysis REVIEW26. Montal M, Mueller P: Formation of bimolecular membranes from lipid monolayers and a study of their electrical properties. Proc. Natl Acad. Sci. USA 69, 35613566 (1972).
27. Neher E, Sakmann B, Steinbach JH: The extracellular patch clamp: a method for resolving currents through individual open channels in biological membranes. Pflgers Arch. 375, 219228 (1978).
28. Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ: Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflgers Arch. 391, 85100 (1981).
29. Neher E, Sakmann B: Single-channel currents recorded from membrane of denervated frog muscle-fibers. Nature 260, 799802 (1976).
30. Krasilnikov OV, Sabirov RZ, Ternovsky VI, Merzliak PG, Tashmukhamedov BA: The structure of Staphylococcus-aureus -toxin-induced ionic channel. Gen. Physiol. Biophys. 7, 467473 (1988).
31. Song L, Hobaugh MR, Shustak C, Cheley S, Bayley H: Structure of staphylococcal -hemolysin, a heptameric transmembrane pore. Science 274, 18591865 (1996).
32. Szabo I, Bathori G, Tombola F, Brini M, Coppola A, Zoratti M: DNA translocation across planar bilayers containing Bacillus subtilis ion channels. J. Biol. Chem. 272, 2527525282 (1997).
33. Szabo I, Bathori G, Tombola F et al.: Double-stranded DNA can be translocated across a planar membrane containing purified mitochondrial porin. FASEB J. 12, 495502 (1998).
34. Akeson M, Branton D, Kasianowicz JJ, Brandin E, Deamer DW: Microsecond time-scale discrimination among polycytidylic acid, polyadenylic acid, and polyuridylic acid as homopolymers or as segments within single RNA molecules. Biophys. J. 77, 32273233 (1999).
35. Meller A, Nivon L, Brandin E, Golovchenko J, Branton D: Rapid nanopore discrimination between single polynucleotide molecules. Proc. Natl Acad. Sci. USA 97, 10791084 (2000).
36. Khulbe PK, Mansuripur M, Gruener R: DNA translocation through -hemolysin nanopores with potential application to macromolecular data storage. J. Appl. Phys. 97, 104317 (2005).
37. Wang H, Dunning JE, Huang APH, Nyamwanda JA, Branton D: DNA
unveiled by single-molecule electrophoresis. Proc. Natl Acad. Sci. USA 101, 1347213477 (2004).
38. Math J, Aksimentiev A, Nelson DR, Schulten K, Meller A: Orientation discrimination of single-stranded DNA inside the -hemolysin membrane channel. Proc. Natl Acad. Sci. USA 102, 1237712382 (2005).
39. Butler TZ, Gundlach JH, Troll MA: Determination of RNA orientation during translocation through a biological nanopore. Biophys. J. 90, 190199 (2006).
40. Meller A, Branton D: Single molecule measurements of DNA transport through a nanopore. Electrophoresis 23, 25832591 (2002).
41. Henrickson SE, Misakian M, Robertson B, Kasianowicz JJ: Driven DNA transport into an asymmetric nanometer-scale pore. Phys. Rev. Lett. 85, 30573060 (2000).
42. Nakane J, Akeson M, Marziali A: Evaluation of nanopores as candidates for electronic analyte detection. Electrophoresis 23, 25922601 (2002).
43. Meller A, Nivon L, Branton D: Voltage-driven DNA translocations through a nanopore. Phys. Rev. Lett. 86, 34353438 (2001).
44. Muthukumar M: Polymer translocation through a hole. J. Chem. Phys. 111, 1037110374 (1999).
45. Lubensky DK, Nelson DR: Driven polymer translocation through a narrow pore. Biophys. J. 77, 18241838 (1999).
46. Jeon TJ, Malmstadt N, Schmidt JJ: Hydrogel-encapsulated lipid membranes. J. Am. Chem. Soc. 128, 4243 (2006).
47. Kasianowicz JJ, Henrickson SE, Weetall HH, Robertson B: Simultaneous multianalyte detection with a nanometer-scale pore. Anal. Chem. 73, 22682272 (2001).
48. Chandler EL, Smith AL, Burden LM, Kasianowicz JJ, Burden DL: Membrane surface dynamics of DNA-threaded nanopores revealed by simultaneous single-molecule optical and ensemble electrical recording. Langmuir 20, 898905 (2004).
49. Nakane J, Wiggin M, Marziali A: A nanosensor for transmembrane capture and identification of single nucleic acid molecules. Biophys. J. 87, 615621 (2004).
50. Tropini C, Marziali A: Multi-nanopore force spectroscopy for DNA analysis. Biophys. J. 92, 16321637 (2007).
51. Sauer-Budge AF, Nyamwanda JA, Lubensky DK, Branton D: Unzipping kinetics of double-stranded DNA in a nanopore. Phys. Rev. Lett. 90, 238101 (2003).
52. Math J, Visram H, Viasnoff V, Rabin Y, Meller A: Nanopore unzipping of individual DNA hairpin molecules. Biophys. J. 87, 32053212 (2004).
53. Math J, Arinstein A, Rabin Y, Meller A: Equilibrium and irreversible unzipping of DNA in a nanopore. Europhys. Lett. 73, 128134 (2006).
54. Bates M, Burns M, Meller A: Dynamics of DNA molecules in a membrane channel probed by active control techniques. Biophys. J. 84, 23662372 (2003).
55. Hornblower B, Coombs A, Whitaker RD et al.: Single-molecule analysis of DNAprotein complexes using nanopores. Nat. Methods 4, 315317 (2007).
56. Snchez-Quesada J, Saghatelian A, Cheley S, Bayley H, Ghadiri MR: Single DNA rotaxanes of a transmembrane pore protein. Agnew. Chem. Int. Ed. 43, 30633067 (2004).
57. Ashkenasy N, Snchez-Quesada J, Bayley H, Ghadiri MR: Recognizing a single base in an individual DNA strand: a step toward DNA sequencing in nanopores. Agnew. Chem. Int. Ed. 44, 14011404 (2005).
58. Vercoutere W, Winters-Hilt S, Olsen H, Deamer D, Haussler D, Akeson M: Rapid discrimination among individual DNA hairpin molecules at single-nucleotide resolution using an ion channel. Nat. Biotechnol. 19, 248252 (2001).
59. Winters-Hilt S, Vercoutere W, DeGuzman VS, Deamer D, Akeson M, Haussler D: Highly accurate classification of WatsonCrick basepairs on termini of single DNA molecules. Biophys. J. 84, 967976 (2003).
60. Winters-Hilt S, Akeson M: Nanopore cheminformatics. DNA Cell Biol. 23, 675683 (2004).
61. Vercoutere WA, Winters-Hilt S, DeGuzman VS et al.: Discrimination among individual WatsonCrick base pairs at the termini of single DNA hairpin molecules. Nucleic Acids Res. 31, 13111318 (2003).
62. DeGuzman VS, Lee CC, Deamer DW, Vercoutere WA: Sequence-dependent gating of an ion channel by DNA hairpin molecules. Nucleic Acids Res. 34, 64256437 (2006).
63. Iqbal R, Landry M, Winters-Hilt S: DNA molecule classification using feature primitives. BMC Bioinformatics 7(Suppl. 2), S15 (2006).
64. Landry M, Winters-Hilt S: Analysis of nanopore detector measurements using machine learning methods, with application to single-molecule kinetic analysis. BMC Bioinformatics (2007) (In press).479future science groupfuture science group www.futuremedicine.com
heterogeneity and phosphorylation
-
REVIEW Healy 65. Churbanov A, Baribault C, Winters-Hilt S: Duration learning for nanopore ionic flow blockade analysis. BMC Bioinformatics (2007) (In press).
66. Winters-Hilt S, Landry M, Akeson M et al.: Cheminformatics methods for novel nanopore analysis of HIV DNA termini. BMC Bioinformatics 7(Suppl. 2), S22 (2006).
67. Winters-Hilt S, Davis A, Amin I, Morales E: Preliminary nanopore cheminformatics of protein binding to non-terminal and terminal DNA regions: analysis of transcription factor binding and retroviral DNA terminus dynamics. BMC Bioinformatics (2007) (In press).
68. Thomson K, Amin I, Morales E, Winters-Hilt S: Preliminary nanopore cheminformatics analysis of aptamer-target binding strength. BMC Bioinformatics (2007) (In press).
69. Winters-Hilt S: The -hemolysin nanopore transduction detector: single-molecule binding studies and immunological screening of antibodies and aptamers. BMC Bioinformatics (2007) (In press).
70. Feynman RP: Simulating physics with computers. Int. J. Theor. Phys. 21, 467488 (1982).
71. DiVincenzo DP: Quantum computation. Science 270, 255261 (1995).
72. Howorka S, Cheley S, Bayley H: Sequence-specific detection of individual DNA strands using engineered nanopores. Nat. Biotechnol. 19, 636639 (2001).
73. Howorka S, Movileanu L, Braha O, Bayley H: Kinetics of duplex formation for individual DNA strands within a single protein nanopore. Proc. Natl Acad. Sci. USA 98, 1299613001 (2001).
74. Howorka S, Bayley H: Probing distance and electrical potential within a protein pore with tethered DNA. Biophys. J. 83, 32023210 (2002).
75. Aksimentiev A, Schulten K: Imaging -hemolysin with molecular dynamics: ionic conductance, osmotic permeability, and the electrostatic potential map. Biophys. J. 88, 37453761 (2005).
76. Astier Y, Braha O, Bayley H: Toward single molecule DNA sequencing: Direct identification of ribonucleoside and deoxyribonucleoside 5-monophosphates by using an engineered protein nanopore equipped with a molecular adapter. J. Am. Chem. Soc. 128, 17051710 (2006).
77. Jett JH, Keller RA, Martin JC et al.: High-speed DNA sequencing: an approach based upon fluorescence detection of single molecules. J. Biomol. Struct. Dyn. 7, 301309 (1989).
78. White RJ, Zhang B, Daniel S et al.: Ionic conductivity of the aqueous layer separating a lipid bilayer membrane and a glass support. Langmuir 22, 1077710783 (2006).
79. Kang XF, Cheley S, Rice-Ficht AC, Bayley H: A storable encapsulated bilayer chip containing a single protein nanopore. J. Am. Chem. Soc. 129, 47014705 (2007).
80. Li J, Stein D, McMullan C, Branton D, Aziz MJ, Golovchenko JA: Ion beam sculpting on the nanoscale. Nature 412, 166169 (2001).
81. Kim MJ, Wanunu M, Bell DC, Meller A: Rapid fabrication of uniformly sized nanopores and nanopore arrays for parallel DNA analysis. Adv. Mater. 18, 31493153 (2006).
82. Wu S, Park SR, Ling XS: Lithography-free formation of nanopores in plastic membranes using laser heating. Nano Lett. 6, 25712576 (2006).
83. Park SR, Peng HB, Ling XS: Fabrication of nanopores in silicon chips using feedback chemical etching. Small 3, 116119 (2007).
84. Healy K, Schiedt B, Morrison AP: A review of solid-state nanopore technologies for nanopore-based DNA analysis. Nanomedicine (In Press).
85. Li J, Gershow M, Stein D, Brandin E, Golovchenko JA: DNA molecules and configurations in a solid-state nanopore microscope. Nat. Mater. 2, 611615 (2003).
86. Chang H, Kosari F, Andreadakis G, Alam MA, Vasmatzis G, Bashir R: DNA-mediated fluctuations in ionic current through silicon oxide nanopore channels. Nano Lett. 4, 15511556 (2004).
87. Ho C, Qiao R, Heng JB et al.: Electrolytic transport through a synthetic nanometer-diameter pore. Proc. Natl Acad. Sci. USA 102, 1044510450 (2005).
88. Storm AJ, Chen JH, Zandbergen HW, Dekker C: Translocation of double-strand DNA through a silicon oxide nanopore. Phys. Rev. E 71, 051903 (2005).
89. Heng JB, Ho C, Kim T et al.: Sizing DNA using a nanometer-diameter pore. Biophys. J. 87, 29052911 (2004).
90. Heng JB, Dimitrov V, Grinkova YV et al.: The detection of DNA using a silicon nanopore. In: IEDM03 Technical Digest. IEEE International, Washington, DC, USA 32.2.132.2.4 (2003).
91. Fologea D, Gershow M, Ledden B, McNabb DS, Golovchenko JA, Li J: Detecting single stranded DNA with a solid state nanopore. Nano Lett. 5, 19051909 (2005).
92. Harrell CC, Choi Y, Horne LP, Baker LA, Siwy ZS, Martin CR: Resistive-pulse DNA detection with a conical nanopore sensor. Langmuir 22, 1083710843 (2006).
93. Chen P, Gu JJ, Brandin E, Kim YR, Wang Q, Branton D: Probing single DNA molecule transport using fabricated nanopores. Nano Lett. 4, 22932298 (2004).
94. Heng JB, Aksimentiev A, Ho C et al.: Stretching DNA using the electric field in a synthetic nanopore. Nano Lett. 5, 18831888 (2005).
95. Heng JB, Aksimentiev A, Ho C et al.: The electromechanics of DNA in a
![Nucleosid * DNA polymerase { ΙΙΙ, Ι } * Nuclease { endonuclease, exonuclease [ 5´,3´ exonuclease]} * DNA ligase * Primase.](https://static.fdocument.org/doc/165x107/56649cab5503460f9496ce53/nucleosid-dna-polymerase-nuclease-endonuclease-exonuclease.jpg)