Lupus nephritis: Nucleosomes trapped by aberrantly expressed laminin-β1
Transcript of Lupus nephritis: Nucleosomes trapped by aberrantly expressed laminin-β1

NATURE REVIEWS | RHEUMATOLOGY VOLUME 9 | DECEMBER 2013
Nature Reviews Rheumatology 9, 698 (2013); published online 19 November 2013; doi:10.1038/nrrheum.2013.181
RESEARCH HIGHLIGHTS
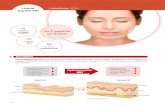
but not laminin‑11). In addition, these interactions can be visualized using immuno‑EM on the GBM from patients with LN to show that laminin‑β1 and nucleosomes form stable complexes that localize to EDDs next to glomerular capillary membranes.
TGF‑β1, which is expressed in immature glomeruli, is downregulated during development and is not found in healthy mature glomeruli. However, TGF‑β1 has previously been shown to induce the aberrant expression of laminin‑1 in the kidney during infection and inflammation, and transgenic mice that produce TGF‑β1 in their kidneys switch from laminin‑β2 to laminin‑β1 production. In this study, the investigators use immuno‑EM to show that TGF‑β1 is expressed in the mesangium and by podocytes during the course of LN. Furthermore, in patients with LN, TGF‑β1 is also trapped within EDDs, although the ligand is unknown.
The authors propose that, in patients with LN, local production of TGF‑β1 from podocytes and mesangial cells
LUPUS NEPHRITIS
Nucleosomes trapped by aberrantly expressed laminin-β1drives the aberrant production of laminin‑β1 chains. Laminin‑1 then colocalizes with nucleosomes in EDDs within the GBM. These trapped nucleosomes are targets for nephritogenic autoantibodies that drive T‑cell‑dependent autoimmune responses.
Importantly, these data support the ‘planted antigen hypothesis’ (that anti‑DNA antibodies form deposits in the kidney by binding to either DNA or nucleosomes). Understanding the early pathogenic mechanisms of LN, and the molecules involved, will be crucial for the development of novel therapeutic strategies in the future.
Bryony Jones
Nucleosomes—key autoantigens involved in the pathogenesis of lupus nephritis (LN)—bind to aberrantly expressed laminin‑β1 and localize to electron‑dense deposits (EDDs) within the glomerular basement membrane (GBM) in patients with LN, according to the authors of a new study. In addition, the researchers report that local production of transforming growth factor (TGF)‑β1 might drive the abnormal expression of laminin‑β1 chains.
Laminins are multidomain trimeric proteins (containing α, β and γ chains) that are mostly found in basement membranes.
In the GBM, laminin‑1 is expressed during development but is
replaced by laminin‑11 in the mature GBM. Using surface
plasmon resonance interaction analysis and electron microscopy (EM),
the researchers found that nucleosomes bind with high
affinity to laminins that contain β1 chains (such as laminin‑1,
Original article Olin, A. I. et al. Pathogenic mechanism in lupus nephritis—nucleosomes bind aberrant laminin-β1 with high affinity, and co-localize in the electron dense deposits. Arthritis Rheum. doi:10.1002/art.38250
NPG
© 2013 Macmillan Publishers Limited. All rights reserved




![Resonance Why Resonance?stevenj/18.369/spring16/TCMT.pdf5/3/12 1 420 nm [ Notomi et al. (2005). ] Resonance an oscillang mode trapped for a long me in some volume (of light, sound,](https://static.fdocument.org/doc/165x107/5e742fb47ad7410ec85993a4/resonance-why-resonance-stevenj18369spring16tcmtpdf-5312-1-420-nm-notomi.jpg)