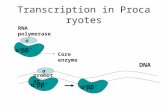
RNA polymerase σ α 2 ββ’ Core enzyme σ promoter DNA α 2 ββ’ Transcription in Procaryotes.
Enzyme 2 protein_structure
-
Upload
sohailvora2457 -
Category
Education
-
view
127 -
download
0
description
Transcript of Enzyme 2 protein_structure

ENZYMES-2
Protein structure


Dipeptide

Polypeptide
Tripeptide
Tetrapeptide
Pentapeptide
Oligopeptide

SINGLE BOND
DOUBLE BOND
It do not allow free rotation of functional
attached to it
It allow free rotation of functional attached to it

Single bondSi
ngle
bon
d
Partial Double bond

• C and N atoms participate in a π bond.
• Which makes lone e- pair on the oxygen.
• There is no free rotation around C-N bond (Peptide bond).

Consequences of Peptide bond as Partial double bond
1. It restricts free rotation around the peptide bond
2. Six atoms of peptide bond forms amide plane.
3. C−N bond length -----------------> 0.133 nm Normal C−N bond lengths------> 0.145 nm Typical C=N bonds----------------> 0.125 nm
4. The peptide bond is estimated to have 40% double-bond character.




Protein Multimeric Homomultimeric
Heteromultimeric
Monomeric
Protomer

Primary Structure

Secondary structure
α-Helixβ-Sheet

STERIC HINDERENCE

STERIC HINDERENCE

STERIC HINDERENCE

STERIC HINDERENCE

Ramachandran Plot
It show the distribution of allowed values in a protein or in a family of proteins.


α-Helix

1. One turn of the helix represents 3.6 a.a. residues.
2. Each amino acid residue extends 1.5 Å3. With 3.6 residues per turn i.e. 3.6 × 1.5 Å = 5.4 Å
This is translation distance/pitch 4. All H-bonds are parallel to helix5. All the carbonyl groups points in one direction6. N-H groups are pointing in the opposite
direction.

Each peptide bond have dipole moment.
Dipole moment is because of N-H and C=O
Such groups lie as helix axis.
Therefore Helix has dipole moment.
N-terminus have partial positive charge & C-terminus have partial negative charge.

β-Sheet
0.347 nm
0.325 nm


• H bonds in this structure are interstrand (not intrastrand)
• Parallel sheets are large(more than five strands).
• Antiparallel sheets have few strands (not more than two)
Hydrophobic side chains
Hydrophobic side chains
Hydrophobic side chains

β-turn

β-turn

Tertiary structure

THE END