C h a p t e rC h a p t e r C h a p t e rC h a p t e r 22 Nuclear Chemistry Brain images with 123...
-
Upload
kathryn-farmer -
Category
Documents
-
view
227 -
download
1
Transcript of C h a p t e rC h a p t e r C h a p t e rC h a p t e r 22 Nuclear Chemistry Brain images with 123...

C h a p t e rC h a p t e r 2222Nuclear ChemistryNuclear Chemistry
Brain images with 123I-labeled (γ-emitter) compound

Chapter 22 Slide 2
Use of 131I (β-emitter) in detecting Hyper- or hypo- thyroidism

Chapter 22 Slide 3
© 2003 John Wiley and Sons Publishers
Henri Becquerel March 1, 1896 :

Chapter 22 Slide 4
Nuclear Reactions 01Nuclear Reactions 01
Animation

Chapter 22 Slide 5
Alpha Decay:Alpha Decay:

Chapter 22 Slide 6
Beta DecayBeta Decay
A beta particle Is an electron
emitted from the nucleus.
Forms when a neutron in the nucleus breaks down.1n 0e + 1P0 -1 1

Chapter 22 Slide 7
Write the nuclear equation for the beta decay of Co-60.
60Co 27
Learning CheckLearning Check

Chapter 22 Slide 8
Write the nuclear equation for the beta decay of Co-60.
60Co 60Ni + 0e 27 28 1
beta particle
SolutionSolution

Chapter 22 Slide 9
Positron Emission:Positron Emission:
Loss of a positron (a particle that has the same mass as but opposite charge than an electron)
e01
C116
B115 + e0
1

Chapter 22 Slide 10
• Gamma radiation is energy emitted from an unstable nucleus indicated by m.
• In a nuclear equation for gamma emission, the mass number and the atomic number are the same.
99mTc 99Tc + 43 43
Gamma RadiationGamma Radiation

Chapter 22 Slide 11
Electron Capture (K-Capture)Electron Capture (K-Capture)
Addition of an electron to a proton in the nucleusAs a result, a proton is transformed into a neutron.
p11 + e0
−1 n1
0

Chapter 22 Slide 12
• Alpha () Radiation: Are helium nuclei, that contain two protons and two neutrons.
• Alpha () emission reduces the mass number by 4 and the atomic number by 2.
242He
Nuclear Reactions 01Nuclear Reactions 01

Chapter 22 Slide 13
Balancing Nuclear Equations
1. Conserve mass number (A).
The sum of protons plus neutrons in the products must equal the sum of protons plus neutrons in the reactants.
1n0U23592 + Cs138
55 Rb9637
1n0+ + 2
235 + 1 = 138 + 96 + 2x1
2. Conserve atomic number (Z) or nuclear charge.
The sum of nuclear charges in the products must equal the sum of nuclear charges in the reactants.
1n0U23592 + Cs138
55 Rb9637
1n0+ + 2
92 + 0 = 55 + 37 + 2x0

Chapter 22 Slide 14
212Po decays by alpha emission. Write the balanced nuclear equation for the decay of 212Po.
4He242oralpha particle -
212Po 4He + AX84 2 Z
212 = 4 + A A = 208
84 = 2 + Z Z = 82
212Po 4He + 208Pb84 2 82
p11 + e0
−1 n1
0

Chapter 22 Slide 16
Nuclear Reactions 06Nuclear Reactions 06
• Write balanced equations for:
1. Alpha emission from curium-242
2. Beta emission from magnesium-28
3. Positron emission from xenon-118
4. Electron capture by polonium-204
• What particle is produced by decay of thorium-214 to radium-210?

Chapter 22 Slide 17
Radioactive Decay Rates 01Radioactive Decay Rates 01

Chapter 22 Slide 18
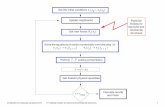
• Radioactive decay is kinetically a first-order process.
Decay Rate = k x N
The integrated form of the first-order rate law is:
lnNtN0
kt
Radioactive Decay Rates 01Radioactive Decay Rates 01
N is number of radio active nuclei in the sample
ln[A]0
[A]
= k t

[A] = k1 . Nt
Chapter 22 Slide 19
Amount of Radioactive Material Remainig and half lifeAmount of Radioactive Material Remainig and half life
ln[A]0
[A]= k t
t½ln2k
= K ln2t1/2
=
ln[A]0
[A]= tln2
t1/2
)/(2lnln 1/20 ttN
N
t
[A]0 = k1 . N0
)/(2lnln 1/2
0tt
N
Nt

Radioactive Decay RatesRadioactive Decay Rates
Radioactive decay is a first-order process.

Chapter 22 Slide 21
Radioactive Decay Rates 02Radioactive Decay Rates 02
• Half-Life: Radioactive decay is characterized by a half-life, t1/2, the time required for the number of radioactive nuclei in a sample to drop to half its initial value.
t12ln 2k

Chapter 22 Slide 22
Radioactive Decay Rates 03Radioactive Decay Rates 03

Chapter 22 Slide 23

Chapter 22 Slide 24

Chapter 22 Slide 25

Chapter 22 Slide 26
lnNtN0
kt
How do you measure rate constant k ?

Chapter 22 Slide 27

Chapter 22 Slide 28

Chapter 22 Slide 29

Chapter 22 Slide 30
0.1813 /day

Chapter 22 Slide 31

Chapter 22 Slide 32

Chapter 22 Slide 33

Chapter 22 Slide 34

Chapter 22 Slide 35

Chapter 22 Slide 36

Chapter 22 Slide 37

Chapter 22 Slide 38

Chapter 22 Slide 39

Chapter 22 Slide 40

Chapter 22 Slide 41

Chapter 22 Slide 42

Chapter 22 Slide 43

Chapter 22 Slide 44
Carbon Dating 01Carbon Dating 01
Carbon-14 is produced in the upper atmosphere by
the bombardment of nitrogen atoms with neutrons:
Radioactive 14CO2 is produced, which mixes with
ordinary 12CO2 and is taken up by plants during
photosynthesis.
147
N + 10
n 146
C + 11
H

Chapter 22 Slide 45
Radiocarbon Dating
14N + 1n 14C + 1H7 160
14C 14N + 0 67 -1 t½ = 5730 years
Uranium-238 Dating
238U 206Pb + 8 4 + 6 092 -182 2 t½ = 4.51 x 109 years

Chapter 22 Slide 46
Carbon Dating 02Carbon Dating 02
• During an organism’s life, 14CO2 and 12CO2 are in a dynamic equilibrium at a ratio of 1 part in 1012.
• When an organism dies, the 14C/12C ratio decreases as 14C undergoes decay to 14N.
• Measuring the 14C/12C ratio determines the age of the sample with a high degree of certainty.
• Ages of 1000–20,000 years are commonly determined. The half-life for 14C is 5730 years.

Chapter 22 Slide 47
Carbon Dating 04Carbon Dating 04
• The carbon-14 decay rate of a sample obtained from
a young live tree is 0.260 disintegrations s–1 g–1. or
15.6 counts per minutes.
• Another sample prepared from an archaeological
excavation gives a decay rate of 0.186
disintegrations s–1 g–1.
• What is the age of the object?
)/(2lnln 1/2
0tt
N
Nt

Nuclear StabilityNuclear Stability

Chapter 22 Slide 49
Neutron-Proton RatiosNeutron-Proton Ratios
• Any element with more than one proton (i.e., anything but hydrogen) will have repulsions between the protons in the nucleus.
• A strong nuclear force helps keep the nucleus from flying apart.
• As the nuclei get heavier more neutron is needed to provide a stable nucleus.

Chapter 22 Slide 50
Neutron-Proton RatiosNeutron-Proton Ratios
• Neutrons play a key role stabilizing the nucleus.
• Therefore, the ratio of neutrons to protons is an important factor.

Chapter 22 Slide 51
Neutron-Proton RatiosNeutron-Proton Ratios
For smaller nuclei (Z 20) stable nuclei have a neutron-to-proton ratio close to 1:1.

Chapter 22 Slide 52
Neutron-Proton RatiosNeutron-Proton Ratios
As nuclei get larger, it takes a greater number of neutrons to stabilize the nucleus.

Chapter 22 Slide 53
Stable NucleiStable Nuclei
The shaded region in the figure shows what nuclides would be stable, the so-called belt of stability.

Chapter 22 Slide 54
Stable NucleiStable Nuclei
• Nuclei above this belt have too many neutrons.
• They tend to decay by emitting beta particles.
1n 0e + 1P0 -1 1

Chapter 22 Slide 55
Stable NucleiStable Nuclei• Nuclei below the belt have
too many protons.• They tend to become more
stable by positron emission.
212Po 4He + 208Pb84 2 82
C116
B115 + e0
1
p11 + e0
−1 n1
0
Alpha emission
or electron capture:

Chapter 22 Slide 56
Nuclear Stability 05Nuclear Stability 05
• Radioactive products of a radioactive decay will undergo further disintegration.
• Some nuclei undergo a whole series of disintegrations called a decay series, leading to nonradioactive species.

Chapter 22 Slide 57
Binding energy 01Binding energy 01
• Since neutrons act as “glue” by overcoming proton–proton repulsions, the strength of these forces should be measurable.
• However, the activation energy required to force elementary particles close enough for reaction is very high and requires temperatures of about 107 K.
• Using Einstein’s equation ∆E = ∆mc2, we can attempt to calculate energies.

Chapter 22 Slide 58
Binding energy 02Binding energy 02
• Consider the formation of a helium-4 nucleus:Total theoretical mass of 2n + 2p = 4.031 88 amu
Observed mass of helium-4 nucleus = 4.001 50 amu
Mass difference = 0.030 38 amu
• Mass difference is called the mass defect of the nucleus. It results from combination of protons and neutrons. It is converted to energy during reaction and is a direct measure of nucleon binding energy.

Chapter 22 Slide 59
Energy Changes 03Energy Changes 03
• Using the Einstein equation, we can calculate the binding energy for a helium-4 nucleus:
• The mass defect = 0.030 38 amu = 0.030 38 g/mol = 3.038 x 10–5
kg/mol.
• ∆E = ∆mc2 = (3.038 x 10–5 kg/mol) (3.00 X 108 m/s)2 (1J/(1 Kg.m2/s2)
(1KJ/1000J)= 2.73 x 109 kJ/mol. (released energy)
(H2(g) + 1/2 O2 -> H2O (l), ΔH = -2.858 x 10-2 kJ/mol)
• The binding energy for helium-4 nucleus is 2.73 x 109 kJ/mol. Which means that 2.73 x 109 kJ/mol is released when helium-4 nucleus formed.
Mass loss of sun is 1010 Kg/sec, in 100 years it loses 6.6 PPT

Chapter 22 Slide 60
Energy Changes 04Energy Changes 04
• Binding Energies are usually expressed on a per–nucleon basis using the electron volt (eV) as the energy unit.
• 1 eV = 1.60 x 10–19 J and 1 MeV = 1.60 x 10–13 J.
• Helium-4 binding energy:
nMeV/nucleo 7.08 Energy binding 4He
nucleons 4
nucleus 1
J101.60
1MeV
nuclei/mol106.022
J/mol102.73Energy binding 4He
1323
12

Chapter 22 Slide 61

Chapter 22 Slide 62

Chapter 22 Slide 63

Chapter 22 Slide 64

Chapter 22 Slide 65

Chapter 22 Slide 66
(1J/(1 Kg.m2/s2)

Chapter 22 Slide 67
(1J/(1 Kg.m2/s2)

Chapter 22 Slide 68
(See slide 60)
Helium-6 is radioactive

Chapter 22 Slide 69

Chapter 22 Slide 70

Chapter 22 Slide 71

Chapter 22 Slide 72

Chapter 22 Slide 73

Chapter 22 Slide 74
Nuclear Fission and Fusion 01Nuclear Fission and Fusion 01
• Nuclear Fission is the fragmentation of heavy nuclei to form lighter, more stable ones.

Chapter 22 Slide 75
Nuclear Fission and Fusion 02Nuclear Fission and Fusion 02
• Nuclear Fission is the fragmentation of heavy nuclei to form lighter, more stable ones.
• Neutrons released in the fission of 235U can induce three more fissions, then nine, and so on leading to a chain reaction.
• Critical mass is the mass required for the chain reaction to become self-sustaining.

Chapter 22 Slide 76

Chapter 22 Slide 77

Chapter 22 Slide 78

Chapter 22 Slide 79

Chapter 22 Slide 80

Chapter 22 Slide 81

Chapter 22 Slide 82

Chapter 22 Slide 83
• Fusion involves the combination of small nuclei to form a larger nucleus.
Nuclear FusionNuclear Fusion

Chapter 22 Slide 84
Nuclear FusionNuclear Fusion
Nuclear Fusion
Among the processes thought to occur in the Sun:
H1
2H1
1+ He2
3
H1
1 e1
0H1
1+ H1
2+
H1
1He2
3+ He2
4+ e1
0
He2
3 2He2
3+ He2
4+ H1
1

Chapter 22 Slide 85
Nuclear Fusion 04Nuclear Fusion 04
• Nuclear Fusion is the formation of heavier nuclei by the joining of lighter ones.
• Fusion products are generally not radioactive.
• Fusion requires high energies (temperatures over 107 K) to overcome the nuclear repulsions. The highest temperature obtained in The Large Hadron
Collider LHC (CERN) is 4X1012 • Fusion reactions are also called thermonuclear.http://www.pppl.gov/projects/pages/tftr.html

Chapter 22 Slide 86

Chapter 22 Slide 87

Chapter 22 Slide 88
Nuclear Fission and Fusion 05Nuclear Fission and Fusion 05
• Nuclear Reactors “control” the fission of 235U and use the energy produced to heat water that drives steam turbines.

Composition of the Spent FuelComposition of the Spent Fuel
• The spent nuclear fuel contains about 93% uranium (mostly U-238)
• about 1% plutonium • less than 1% minor actinides (neptunium, americium, and curium)
• 5% fission products

Global Nuclear WastesGlobal Nuclear Wastes
• Typical reactor will generate 20 to 30 tons of high-level nuclear waste annually
• The global volume of spent fuel is ,290,000 tons , and is
growing by approximately 10,000 tons annually.
• Despite billion of dollars of investment in various disposal options, the nuclear industry and governments have failed to come up with a feasible and sustainable solution.

U-238 decay chain (main branch)U-238 decay chain (main branch)
• Uranium-238 (half-life: 4.46 billion years) alpha decay ==>• Thorium-234 (half-life: 24.1 days) beta decay ==>• Protactinium-234m half-life: 1.17 minutes) beta decay ==>• Uranium-234 (half-life: 245,000 years) alpha decay ==>• Thorium-230 (half-life: 75,400 years) alpha decay ==>• Radium-226 (half-life: 1,600 years) alpha decay• ==>• Radon-222 (half-life: 3.82 days) ==> followed by radon
decay products (polonium, bismuth, lead isotopes)

Thorium-232Thorium-232
• Thorium-232 is, like U-238, has its own decay chain
• Dangerous decay products build up relatively quickly in Th-232
• They are thorium-228, actinium-228 (a beta-emitter), radium-228, and radium-224
• Radium-224 gives off radon-220 (which is similar to radon-222)

Repository capacityRepository capacity
• Three isotopes, which are linked through a decay process (Pu241, Am241, and Np237), are the major contributors to the estimated dose for releases from the repository, typically occurring between 100,000 and 1 million years, and also to the long-term heat generation that limits the amount of waste that can be placed in the repository

Composition of the Spent FuelComposition of the Spent Fuel
• The spent nuclear fuel contains about 93% uranium (mostly U-238)
• about 1% plutonium • less than 1% minor actinides (neptunium, americium, and curium)
• 5% fission products

Composition of the Spent FuelComposition of the Spent Fuel
• The spent nuclear fuel contains about 93% uranium (mostly U-238)
• about 1% plutonium • less than 1% minor actinides (neptunium, americium, and curium)
• 5% fission products

Chapter 22 Slide 96
Nuclear Fission & POWERNuclear Fission & POWERNuclear Fission & POWERNuclear Fission & POWER
• Currently* about 103 Currently* about 103
nuclear power plants nuclear power plants
in the U.S. and about in the U.S. and about
442 worldwide.442 worldwide. There
65 currently under
construction
• 17% of the world’s energy 17% of the world’s energy
comes from nuclear.comes from nuclear.
* 12-03-12

Nuclear Fission and FusionNuclear Fission and Fusion

Chapter 22 Slide 98Size of a fission bumbSize of a fission bumb
© 2003 John Wiley and Sons Publishers
Courtesy US Department of Energy

Chapter 22 Slide 99
© 2003 John Wiley and Sons Publishers
The plutonium was produced in Hanford Nuclear reservation

Chapter 22 Slide 100
Nuclear Transmutation 01Nuclear Transmutation 01
• Nuclear Transmutation
is the change of one element
into another.
• Achieved by bombarding
atoms with high-energy
particles in a
particle accelerator.
• Transmutation can synthesize
new elements.

Chapter 22 Slide 101
Nuclear Transmutation 02Nuclear Transmutation 02
• Cyclotrons consist of D-shaped electrodes (dees) with a large, circular magnet above and below the vacuum chamber.
• Particles are accelerated by making the dees alternatively positive and negative.
• When the particles are moving at sufficient velocity they are allowed to escape the cyclotron and strike the target.

Chapter 22 Slide 102
Nuclear TransmutationNuclear Transmutation
Elements beyond 92 Elements beyond 92 (transuranium)(transuranium) made made
starting with an starting with an n,n, reaction reaction
2382389292U + U + 11
00n ---> n ---> 2392399292U + U +
2392399292U U ---> ---> 239239
9393Np + Np + 00-1-1
2392399393Np Np ---> ---> 239239
9494Pu + Pu + 00-1-1

Chapter 22 Slide 103
Nuclear TransmutationNuclear Transmutation
Nuclear Transmutation: The change of one element into another.
Plutonium-241 can be made by bombarding uranium-238 with alpha particles:
Pu94
241 Am95
241+ e-1
0
Plutonium-241 decays into americium-241:
He2
4U92
238+ Pu94
241+ n0
1

Chapter 22 Slide 104
Nuclear TransmutationNuclear Transmutation
Nuclear Transmutation: The change of one element into another.
Cobalt-60 is used in radiation therapy for cancer patients. The overall preparation process can be written as:
2Fe26
58+ Co27
60+ e-1
0n0
1

Chapter 22 Slide 105
Radioisotopes in Medicine• 1 out of every 3 hospital patients will undergo a nuclear
medicine procedure
• 24Na, t½ = 14.8 hr, emitter, blood-flow tracer
• 131I, t½ = 14.8 hr, emitter, thyroid gland activity
• 123I, t½ = 13.3 hr, ray emitter, brain imaging
• 18F, t½ = 1.8 hr, emitter, positron emission tomography
• 99mTc, t½ = 6 hr, ray emitter, imaging agent
Brain images with 123I-labeled compound

Chapter 22 Slide 106
Detecting Radioactivity 01Detecting Radioactivity 01
• Matter is ionized by radiation.
• We can detect radiation by measuring its ionizing properties.
• Ionizing radiation includes particles, particles, rays, X rays, and cosmic rays.
• ray & X rays are high-energy photons ( = 10–8 to 10–11 m). Cosmic rays originate in interstellar space.

Chapter 22 Slide 107
Detecting Radioactivity 02Detecting Radioactivity 02
• A Geiger counter determines the amount of ionization by detecting an electric current.
• A thin window is penetrated by the radiation and causes the ionization of Ar gas.
• The ionized gas carried a charge and so current is produced.
• The current pulse generated when the radiation enters is amplified and counted.

Chapter 22 Slide 108
Detecting Radioactivity 04Detecting Radioactivity 04
• Scintillation counters use a substance called phosphor (sodium iodide & thallium iodide), which emits a flash of light when struck by radiation.
• Flashes can be counted electronically and converted to an electric signal.

Chapter 22 Slide 109
• Radiotracers (radio-labels) are used to follow an element through a chemical reaction.
• Photosynthesis has been studied using 14C-containing carbon dioxide:
• The carbon dioxide is said to be 14C-labeled.
614CO2 + 6H2O 14C6H12O6 + 6O2sunlightchlorophyll
Application of Radioisotopes05Application of Radioisotopes05

Chapter 22 Slide 110
Biological Effects of Radiation 01Biological Effects of Radiation 01
• The penetrating power of radiation is a function of its mass: -rays > -particles >> -particles.
• When ionizing radiation passes through tissue it removes an electron from water to form H2O+ ions.
• The H2O+ ions react with another water molecule to produce H3O+ and a highly reactive •OH radical.
• Free radicals generally undergo chain reactions, producing many radicals in the biomolecules.

Chapter 22 Slide 111
Biological Effects of Radiation 02Biological Effects of Radiation 02
• -rays are particularly harmful
because they penetrate in the
same way as X rays.
• -particles interact with the skin
and -particles interact up to 1
cm into the tissue
• -particles are particularly
dangerous when ingested or
inhaled.

Chapter 22 Slide 112
Radiation MeasurementRadiation Measurement
• The Curie measures the number of atoms that decay in one second. Curie: 1 Ci = 3.7 x 10Curie: 1 Ci = 3.7 x 101010 distintegrations/sdistintegrations/s
• The rad* (radiation absorbed dose) measures the radiation absorbed by the tissues of the body.
• The rem (Roentgen equivalent for man (rem) ) measures the biological damage.
*1 Rad = 2.58 x 10-4 Coulombs /kg air. The exposure rate expresses the rate of charge production per unit mass of air and
is commonly expressed in roentgens per hour (R/h) or milliroentgens per hour (mR/h).

Chapter 22 Slide 113
Biological Effects of RadiationRadiation absorbed dose (rad)
1 rad = 1 x 10-5 J/g of material
Roentgen equivalent for man (rem)
1 rem = 1 rad x Q Quality Factor-ray = 1
= 1 = 20
Curie: 1 Ci = 3.7 x 10Curie: 1 Ci = 3.7 x 101010
distintegrations/sdistintegrations/s
SI unit is the becquerel: SI unit is the becquerel:
Bq = 1 Bq = 1 distintegrations/sdistintegrations/s

Chapter 22 Slide 114
Units of Radiation MeasurementUnits of Radiation Measurement

Chapter 22 Slide 115
Background RadiationBackground Radiation
• A person is exposed to radiation from naturally occurring radioisotopes and medical X rays.

Chapter 22 Slide 116
Biological Effects of Radiation 08Biological Effects of Radiation 08

Chapter 22 Slide 117
Biological Effects of Radiation 07Biological Effects of Radiation 07

Chapter 22 Slide 118
Biological Effects of RadiationBiological Effects of Radiation

Chapter 22 Slide 119
Applications of Nuclear ChemistryApplications of Nuclear Chemistry
The half-life of carbon-14 is 5730 years:
The measured ratio of carbon-14/carbon-12 after death can determine how long ago the organism died.
Dating with Radioisotopes
e-1
0C14
14 N7
14+

Chapter 22 Slide 120
Applications of Nuclear ChemistryApplications of Nuclear Chemistry
Geologic age can be determined by analysis of potassium-40:
Dating with Radioisotopes
e1
0K19
40 Ar18
40+
e-1
0K19
40 Ar18
40+
Potassium-40 has a half life of 1.25 billion years. Mass Spectroscopy is used to measure Ar-40 in a sample of molten rock to calculate the age of the rock.

Bone scan Using Radiactive Technetiun-99
Bone scan