Three-year follow-up of bintrafusp alfa, a bifunctional fusion … · 2020. 3. 31. · Abstract No....
Transcript of Three-year follow-up of bintrafusp alfa, a bifunctional fusion … · 2020. 3. 31. · Abstract No....
-
Abstract No. 1443. Presented at the ESMO Virtual Congress 2020, September 19-21, 2020
Three-year follow-up of bintrafusp alfa, a bifunctional fusion protein targeting TGF-β and PD-L1, for second-line (2L) treatment of non-small cell lung cancer (NSCLC)
L. Paz-Ares1, T. M. Kim2, D. Vicente3, E. Felip4, D. H. Lee5, K. H. Lee6, C.-C. Lin7, M. J. Flor8, M. Di Nicola9, R. M. Alvarez10, C. Helwig11, L. S. Ojalvo12, J. L. Gulley13, B. C. Cho141Hospital Universitario 12 de Octubre, Madrid, Spain; 2Seoul National University Hospital, Seoul, Republic of Korea; 3Hospital Universitario Virgen Macarena, Seville, Spain; 4Vall d’Hebron University Hospital and Institute of Oncology, UVic-UCC, IOB-Quiron, Barcelona, Spain; 5Asan Medical Center, University of Ulsan College of Medicine Seoul, Seoul, Republic of Korea; 6Chungbuk National University Hospital, Chungcheongbuk-Do, Republic of Korea; 7National Taiwan University Hospital, Taipei, Taiwan; 8Hospital Universitario Virgen del Rocío, Seville, Spain; 9Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy; 10Gregorio Marañon Hospital, Madrid, Spain; 11Merck KGaA, Darmstadt, Germany; 12EMD Serono Research & Development Institute, Inc., Billerica, MA, USA; a business of Merck KGaA, Darmstadt, Germany; 13National Cancer Institute, National Institutes of Health, Bethesda, MD, USA; 14Yonsei Cancer Center, Yonsei University College of Medicine, Seoul, Republic of Korea
Poster No. 1272P
BACKGROUNDNSCLC• Lung cancer is the leading cause of cancer-related deaths worldwide, and the most
common type of lung cancer is NSCLC1,2
• TGF-β plasma levels are increased in patients with NSCLC compared with levels in healthy volunteers3
• Activation of the TGF-β pathway promotes epithelial-mesenchymal transition (EMT), which is associated with tumor progression, fibrosis, and drug resistance4-6
• Objective response rates (ORRs) of 12% to 20% have been reported with PD-(L)1 inhibitors in patients with metastatic NSCLC in the 2L or later setting, depending on PD-L1 expression7-9
– In patients with PD-L1–unselected tumors, the median progression-free survival (PFS) ranged from 2.3 to 3.5 months, and the median overall survival (OS) ranged from 9.2 to 13.8 months, demonstrating the need for improved treatment options9-11
Bintrafusp alfa• Bintrafusp alfa is a first-in-class bifunctional fusion protein composed of the
extracellular domain of the TGF-βRII receptor to function as a TGF-β “trap” fused to a human IgG1 antibody blocking PD-L1 (Figure 1)
– The bifunctional nature of bintrafusp alfa might allow for colocalized, simultaneous inhibition of two nonredundant immunosuppressive pathways, TGF-β and PD-L1, within the tumor microenvironment12
• Previous results from a global phase 1 study (NCT02517398) reported clinical efficacy and a manageable safety profile in patients with advanced NSCLC who received bintrafusp alfa 1200 mg in the 2L setting13,14
– Here, we present efficacy and safety data for 3 years of follow-up in this cohort of patients with advanced NSCLC receiving bintrafusp alfa
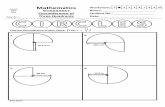
Figure 1. Proposed mechanism of action of bintrafusp alfa
Fibroblast
CAF
Fibrosis and impaireddrug access
EMT (leading to metastasis and resistanceto therapy [including checkpoint inhibition])
Tumor angiogenesis
Tumor cells
PD-L1
Bintrafuspalfa
PD-1
T cell
Suppression of immune response
NK cell
TAMDendritic
cell
CytotoxicT cell
Tumor cellsMesenchymal-like
tumor cell
TGF-β “trap” moiety sequesters TGF-β to block downstream signaling
Anti–PD-L1 mAb moietyblocks PD-L1 interactions with PD-1
TGF-β*
CAF, cancer-associated fibroblast; NK, natural killer; TAM, tumor-associated macrophage.
*Tumor cells are also a major source of TGF-β in the microenvironment.
METHODS• NCT02517398 is a phase 1, open-label trial of bintrafusp alfa13
• Patients with advanced NSCLC, unselected for PD-L1 expression, who had disease progression after standard first-line (1L) treatment and received no prior immunotherapy were randomized to receive bintrafusp alfa 500 mg or the recommended phase 2 dose of 1200 mg (n=40 each) every 2 weeks (Q2W) until disease progression, unacceptable toxicity, or trial withdrawal
– Patients who achieved stable disease (SD), complete response (CR), or partial response (PR) with subsequent disease progression may reinitiate treatment for up to 12 months
• The primary objective of this expansion cohort study was to assess best overall response (BOR) per RECIST 1.1, as described previously13
– Key secondary objectives were safety and pharmacokinetics – Exploratory objectives included duration of response (DOR), PFS, and OS
• Tumor cell PD-L1 expression was measured by immunohistochemistry (IHC) using antibody clone 73-10 (PD-L1 IHC 73-10 pharmDx; Dako, Carpinteria, CA, USA); expression levels were categorized as PD-L1 negative (