SOLUTION PROBLEMS - WordPress.com … · Web viewBe sure to write what you know, the formula,...
Transcript of SOLUTION PROBLEMS - WordPress.com … · Web viewBe sure to write what you know, the formula,...

Name ______________________________________________ Period _____
MORE SOLUTION PROBLEMS
Helpful FormulasMolarity (M) = mol solute Molality = mol solute M1V1 = M2V2 ΔT = K df m
liter soln kg solvent
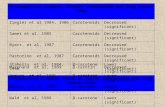
Molal Freezing and Boiling Point Constants (in C°/m )Substance Freezing Point °C Molal Freezing
Point constantBoiling Point °C Molal Boiling
Point constantBenzene 5.533 5.12 80.100 2.53
Nitrobenzene 5.76 6.852 210.8 5.24Water 0.00 1.853 100.000 0.515
Be sure to write what you know, the formula, plug in your numbers with units and calculate the correct answer with units and significant figures.1. Calculate the molarity of 800.0 mL which contains 30.0 g of acetic acid (CH3COOH).
2. Calculate the molarity of 2000.0 mL which contains 49.0 g of phosphoric acid, H3PO4.
3. Calculate the mass of the solute in 750.0 mL of CaCl2 solution which is 0.500 M.
4. Calculate the mass of the solute in 3000.0 mL of a KOH solution which is 2.00 M.
5. How many liters of solution can be made from 2.00 M solution using 80.0 g sodium hydroxide, NaOH?
6. How many liters of solution can be made from 0.500 M solution using 80.0 g sodium hydroxide?
7. Calculate the molality of 1.50 mol NaCH3COO dissolved in 750.0 g of water.
8. Calculate the molality of 3.00 mol H2SO4 dissolved in 1250.0 g of water.

9. Calculate the volume of 12.0M HCl needed to make 500.0mL of a 1.5M HCl solution.
10. Calculate the volume of 17.4M HC2H3O2 needed to make 1.0L of a 3.0M HC2H3O2 solution.
11. What are the freezing and boiling points of a solution that contains 10.0 g naphthalene, C10H8, dissolved in 50.0 g benzene, C6H6?
12. What are the freezing and boiling points of a solution that contains 23.0 g MgSO4 dissolved in 250.0 g water?
13. What is the molecular mass of an organic compound if 16.0 g of the compound when dissolved in 225.0 g nitrobenzene increases the boiling point by 8.56oC?