PETROCHEMICALS Ineos Nova to get rights to Sterling's styrene operations
Transcript of PETROCHEMICALS Ineos Nova to get rights to Sterling's styrene operations
NEWS OF THE WEEK
CATALYSIS ACROSS A RING ORGANIC SYNTHESIS: Catalytic
transannular Diels-Alder reaction is highly selective
Β ONDING SPECIFIC carbon centers across steri-cally congested rings can be nearly impossible without the right catalyst.
Chemistry professor Eric N. Jacobsen and graduate student Emily P. Balskus at Harvard University report the first transannular Diels-Alder reaction to occur with the help of a highly selective asymmetric catalyst. They used it to catalyze a key step in the total synthesis of a sesquiterpene natural product (Science 2007,317,1736).
Organic chemist Erik J. Sorensen at Princeton University comments that the work '"will stimulate other creative applications of this method in complex chemical synthesis." In some instances, he adds, the chiral catalyst can even override the intrinsic selectivity bias of a substrate.
Thomas R. Hoye, a synthetic organic chemist at the University of Minnesota, Twin Cities, says that "the results serve as an elegant demonstration of the power of synthetic design that can follow from insightful analysis of critical structural details."
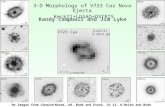
Oxazaborolidine-based Lewis acid compounds are known to effectively catalyze intermolecular and acyclic intramolecular Diels-Alder reactions. To achieve high selectivity for transannular reactions, Balskus and Jacobsen synthesized and tested various derivatives of these catalysts. An ortho-fluorinated phenyl ring on the boron provided the best conversion and selectivity.
Balskus notes they were careful to choose macrocy-
Ph = phenyl Tf = triflate (CF3S02-) R | N Q CROSSING An
optimized catalyst (in which R = 2-fluorophenyl) mediates this asymmetric transannular Diels-Alder reaction.
Transannular reaction
clic substrates that would be amenable to catalysis but would not spontaneously undergo a transannular Diels-Alder reaction in the absence of a catalyst or at room temperature.
Jacobsen says, "We observed consistently high enantioselectivity, and that makes us very optimistic that this type of chemistry might be applied to a wide range of substrates and maybe even different types of reactions." He and his colleagues started with the Diels-Alder reaction because it is a well-studied reaction and they could build on extensive previous work related to asymmetric catalysis, Jacobsen adds.
After establishing that the catalyst would react with a broad range of macrocycles, the researchers applied their synthetic method to a more elaborate substrate, namely, a sterically congested sesquiterpene natural product called 11,12-diacetoxydrimane. In eight steps from a known ketoaldehyde, they first synthesized the desired substrate for the transannular Diels-Alder reaction. Then, they used the optimized catalyst to convert that intermediate, a silicon-containing macrocy-clic ketone, to a tricyclic compound. Six additional steps led to the desired product.
The Harvard researchers note that the tricyclic compound provides access to other interesting natural products that, due to steric demands, are inaccessible by traditional inter- or intramolecular Diels-Alder reactions.—RACHEL PETKEWICH
PETROCHEMICALS Ineos Nova to get rights to Sterling's styrene operations Nova Chemicals is taking steps to improve the health of its styrenics business. No sooner had the ink dried on the Federal Trade Commission's approval of Nova's styrenics joint venture with Ineos when Nova pulled off another deal.
The Canadian company has secured exclusive rights to styrene production at Sterling Chemical's Texas City, Texas, facility. Nova will transfer the rights to the joint venture, Ineos Nova, when it launches on Oct. 1. Ineos Nova will also pay the $60 million price for the rights. "We believe this is a very significant step
that will accelerate a return to financial health for the styrenics chain," said Nova CEO Jeffrey M. Lipton.
At a meeting for reporters and analysts last week, Lipton revealed that once both deals are done, the joint venture will almost certainly shut the Texas City plant to improve efficiency of its other styrenics operations. The facility has annual styrene capacity of 1.7 billion lb, representing about 11% of North American and 3% of global capacity.
The shutdown, Lipton said, will improve Ineos Nova's financials by about
$30 million per year as the company moves customers to its other plants. He estimates that operating rates of styrene plants worldwide will increase from 88% currently to 9 1 % . In North America, the increase will be from 82% to 93%.
Still to be heard from is the styrenics joint venture being formed by Chevron Phillips Chemical and Dow Chemical. It has received government clearance, and industry observers are hoping that, once complete, those partners will also close some capacity.—WILLIAM STORCK
v'VVVV.CEN-'ONLINE.C'RG 17 SEPT 24, 2007