Oxford Poster
-
Upload
bccunitcell -
Category
Documents
-
view
50 -
download
1
description
Transcript of Oxford Poster

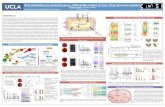
Atomistic Studies on Hydrogen and Helium in Fe
0
0.1
0.2
0.3
0.4
0.5
0.6
0.7
0.8
O1 T1 T2 T4 O1’
ΔE
(eV)
EAMDFT
-2
-1
0
1
2 Molecular Hydrogen and Perfect Bulk Iron
-2
-1
0
1
2 Hydrogen in T-site
-2
-1
0
1
2
-10 -8 -6 -4 -2 0 2E - EF (eV)
Hydrogen at MonovacancyVacancies act as strong traps for hydrogen, however, dissociation of a hydrogen-vacancy pair is more likely than coherent migration
Bulk di�usion is very low barrier, and it may proceed via two competing paths
We must account for zero-point energy (ZPE) corrections
Di�erent environments result in changes to the electronic structure of hydrogen, with delocaliza-tion in space accompanied by delocalization in energy
Di�usion and Electronic Structure of Hydrogen in α-Fe
Saturation of the surface may be followed by molecule formation within the void
Models based on surface absorption sites are being developed to predict when saturation occurs
Due to the tendency to hybridize with Fe, atomic H sticks to the surface of vacancy clusters; molecules are unstable
Saturation
Hydrogen
Hydrogen retention and embrittlement are relevant issues for today’s �ssion reactors and tomorrow’s fusion industry
Atomistic simulations -- density functional theory, molecular dynam-ics, and Monte Carlo -- can be used to give insight into fundamental lattice defect-impurity interactions
This study focuses on the interplay between hydrogen and vacancies in body-centered cubic iron, keeping these questions in mind:
How is the di�usion of hydrogen a�ected by vacancies, and vice versa?
How is the form and behavior of a hydrogen-vacancy cluster a�ected by concentration? size?
Hydrogen + Helium
0 5 10 15 20 25
0
1
2
3
4
5
6
7
8
Hydrogen atoms
Hel
ium
ato
ms
When hydrogen and helium are both present, additional damage to the material can occur; understanding this synergistic interaction will be vital for fusion environments
Although H has only minimal self-attraction in the bulk, He tends to form clusters
Hydrogen is trapped at these clusters, perhaps attracted to the free volume between He and the Fe
Displacement of Fe surrounding cluster (units of a0, MDMC
result)
Hydrogen may assist in helium-induced Frenkel pair ejection
A substitutional He atom can trap up to 8 hydrogen atoms (as opposed to the 6 that a monovacancy can trap), even though the direct attraction between H and He is negligible
For more details, see our paper:
Interplay between hydrogen and vacancies in α-Fe Physical Review B 87, 174103 (2013)
or contact us at: [email protected] [email protected]
We use an iterative method to search for low energy states of defect clusters, consisting of perturbation, minimization, and Monte Carlo selection criteria
Minimization may be handled using di�erent methods:
Empirical interatomic potentials, molecular dynamics, LAMMPS
Density functional theory, SIESTA
Low Energy State Searches
Erin Hayward and Chu Chun FuCEA-Saclay, DEN/DMN, Service de Recherches de Métallurgie Physique