Opinion: A worm's eye view of the immune system: consequences for evolution of human autoimmune...
Transcript of Opinion: A worm's eye view of the immune system: consequences for evolution of human autoimmune...

© 2005 Nature Publishing Group
420 | MAY 2005 | VOLUME 5 www.nature.com/reviews/immunol
P E R S P E C T I V E S
98. Yoshida, H. et al. Expression of α4β7 integrin defines a distinct pathway of lymphoid progenitors committed to T cells, fetal intestinal lymphotoxin producer, NK, and dendritic cells. J. Immunol. 167, 2511–2521 (2001).
AcknowledgementsI thank R. Newberry, R. Mebius, N. Ruddle, M. Poles, D. Guy-Grandand P. Vassalli for discussion and critical reading of the manuscript.I also thank M. Tsuji for constant support and many debates, andD. Littman for introducing me to ROR-γt.
Competing interests statementThe author declares no competing financial interests.
Online links
DATABASESThe following terms in this article are linked online to:Entrez Gene:http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=geneCCL19 | CCL21 | CXCL13 | ICAM1 | IL-7 | IL-7Rα | LT-α1β2 |LT-βR | ROR-γt | TNF | TNFR1 | VCAM1 Access to this interactive links box is free online.
92. Hayday, A., Theodoridis, E., Ramsburg, E. & Shires, J.Intraepithelial lymphocytes: exploring the third way inimmunology. Nature Immunol. 2, 997–1003 (2001).
93. Cheroutre, H. & Madakamutil, L. Acquired and naturalmemory T cells join forces at the mucosal front line.Nature Rev. Immunol. 4, 290–300 (2004).
94. Huang, F. P. et al. A discrete subpopulation of dendriticcells transports apoptotic intestinal epithelial cells to T cell areas of mesenteric lymph nodes. J. Exp. Med.191, 435–444 (2000).
95. Fleeton, M. N. et al. Peyer’s patch dendritic cells processviral antigen from apoptotic epithelial cells in the intestine of reovirus-infected mice. J. Exp. Med. 200, 235–245(2004).
96. Mebius, R. E., Streeter, P. R., Michie, S., Butcher, E. C. &Weissman, I. L. A developmental switch in lymphocytehoming receptor and endothelial vascular addressinexpression regulates lymphocyte homing and permitsCD4+CD3– cells to colonize lymph nodes. Proc. NatlAcad. Sci. USA 93, 11019–11024 (1996).
97. Mebius, R. E. et al. The fetal liver counterpart of adultcommon lymphoid progenitors gives rise to all lymphoidlineages, CD45+CD4+CD3– cells, as well asmacrophages. J. Immunol. 166, 6593–6601 (2001).
65. Fujihashi, K. et al. Interleukin 2 (IL-2) and interleukin 7(IL-7) reciprocally induce IL-7 and IL-2 receptors on γδ T-cell receptor-positive intraepithelial lymphocytes.Proc. Natl Acad. Sci. USA 93, 3613–3618 (1996).
66. Spahn, T. W. et al. Induction of colitis in mice deficientof Peyer’s patches and mesenteric lymph nodes is associated with increased disease severity andformation of colonic lymphoid patches. Am. J. Pathol.161, 2273–2282 (2002).
67. Kaiserling, E. Newly-formed lymph nodes in thesubmucosa in chronic inflammatory bowel disease.Lymphology 34, 22–29 (2001).
68. Fagarasan, S. et al. Critical roles of activation-inducedcytidine deaminase in the homeostasis of gut flora.Science 298, 1424–1427 (2002).
69. Revy, P. et al. Activation-induced cytidine deaminase (AID)deficiency causes the autosomal recessive form of thehyper-IgM syndrome (HIGM2). Cell 102, 565–575 (2000).
70. Yamamoto, M. et al. Role of gut-associatedlymphoreticular tissues in antigen-specific intestinal IgAimmunity. J. Immunol. 173, 762–769 (2004).
71. Takeda, K., Kaisho, T. & Akira, S. Toll-like receptors.Annu. Rev. Immunol. 21, 335–376 (2003).
72. Cario, E. & Podolsky, D. K. Differential alteration inintestinal epithelial cell expression of Toll-like receptor 3(TLR3) and TLR4 in inflammatory bowel disease. Infect. Immun. 68, 7010–7017 (2000).
73. Ortega-Cava, C. F. et al. Strategic compartmentalizationof Toll-like receptor 4 in the mouse gut. J. Immunol.170, 3977–3985 (2003).
74. Abreu, M. T. et al. Decreased expression of Toll-likereceptor-4 and MD-2 correlates with intestinal epithelialcell protection against dysregulated proinflammatory geneexpression in response to bacterial lipopolysaccharide. J. Immunol. 167, 1609–1616 (2001).
75. Monteiro, R. C. & Van De Winkel, J. G. IgA Fc receptors.Annu. Rev. Immunol. 21, 177–204 (2003).
76. Ardavin, C. Origin, precursors and differentiation ofmouse dendritic cells. Nature Rev. Immunol. 3, 582–590(2003).
77. Papadakis, K. A. & Targan, S. R. Role of cytokines in thepathogenesis of inflammatory bowel disease. Annu. Rev.Med. 51, 289–298 (2000).
78. Brenner, O. et al. Loss of Runx3 function in leukocytes is associated with spontaneously developed colitis andgastric mucosal hyperplasia. Proc. Natl Acad. Sci. USA101, 16016–16021 (2004).
79. Carlsen, H. S., Baekkevold, E. S., Johansen, F. E.,Haraldsen, G. & Brandtzaeg, P. B cell attractingchemokine 1 (CXCL13) and its receptor CXCR5 areexpressed in normal and aberrant gut associatedlymphoid tissue. Gut 51, 364–371 (2002).
80. Gommerman, J. L. & Browning, J. L. Lymphotoxin/LIGHT,lymphoid microenvironments and autoimmune disease.Nature Rev. Immunol. 3, 642–655 (2003).
81. Tracey, K. J. & Cerami, A. Tumor necrosis factor: a pleiotropic cytokine and therapeutic target. Annu. Rev. Med. 45, 491–503 (1994).
82. Muramatsu, M. et al. Class switch recombination and hypermutation require activation-induced cytidinedeaminase (AID), a potential RNA editing enzyme. Cell 102, 553–563 (2000).
83. Sansonetti, P. J. War and peace at mucosal surfaces.Nature Rev. Immunol. 4, 953–964 (2004).
84. Mayer, L. & Shao, L. Therapeutic potential of oraltolerance. Nature Rev. Immunol. 4, 407–419 (2004).
85. Wu, H. Y. & Weiner, H. L. Oral tolerance. Immunol. Res. 28, 265–284 (2003).
86. Ruddle, N. H. Lymphoid neo-organogenesis:lymphotoxin’s role in inflammation and development.Immunol. Res. 19, 119–125 (1999).
87. Hjelmstrom, P. Lymphoid neogenesis: de novoformation of lymphoid tissue in chronic inflammationthrough expression of homing chemokines. J. Leukoc.Biol. 69, 331–339 (2001).
88. Moyron-Quiroz, J. E. et al. Role of inducible bronchusassociated lymphoid tissue (iBALT) in respiratoryimmunity. Nature Med. 10, 927–934 (2004).
89. Jacob, E., Baker, S. J. & Swaminathan, S. P. ‘M’ cellsin the follicle-associated epithelium of the humancolon. Histopathology 11, 941–952 (1987).
90. O’Leary, A. D. & Sweeney, E. C. Lymphoglandularcomplexes of the colon: structure and distribution.Histopathology 10, 267–283 (1986).
91. Guy-Grand, D., DiSanto, J. P., Henchoz, P., Malassis-Seris, M. & Vassalli, P. Small bowelenteropathy: role of intraepithelial lymphocytes and ofcytokines (IL-12, IFN-γ, TNF) in the induction of epithelialcell death and renewal. Eur. J. Immunol. 28, 730–744(1998).
A worm’s eye view of the immunesystem: consequences for evolutionof human autoimmune disease
David W. Dunne and Anne Cooke
O P I N I O N
Abstract | Humans and the many parasitesthat we can host have co-evolved overmillions of years. This has been comparedto an arms race in which the immunearmoury of the human has evolved to dealwith potential pathogens and the pathogenhas evolved strategies to evade, and insome cases use, the immune system of the human host. Recently, there have been marked changes in the exposure ofindividuals in the developed world to bothmicroorganisms and metazoan parasites,so the immune stimuli such organismsprovide no longer have a role in our lives.As we discuss here, this is a markedperturbation, and the absence of theassociated immunomodulation might have led to the increased emergence of autoimmune diseases.
Autoimmune disease arises when toleranceto self-tissue breaks down and pathologydevelops. With the exception of rare auto-immune diseases that result from mutations in a single gene, such as autoimmune poly-endocrinopathy candidiasis ectodermaldystrophy (APECED)1, most autoimmunediseases are influenced by variations at severalgenetic loci and by environmental factors.A role for the environment in the development
of most autoimmune diseases is indicated bythe less than 100% concordance of diseasedevelopment in monozygotic twins; forexample, the concordance rate for type 1diabetes in monozygotic twins is only ∼40%(REF. 2). The incidence of some autoimmunediseases, such as type 1 diabetes and systemiclupus erythematosus, has been markedlyincreasing in the developed world on a time-scale that rules out genetic change as anexplanation. In the case of type 1 diabetes,which is a T helper 1 (T
H1)-cell-mediated
autoimmune disease with juvenile onset,this is particularly important, as it was lethaluntil Banting and Best3 discovered insulin, inthe 1920s. This implies that humans haveretained potentially lethal allelic variants ofcertain genes, either because they have histori-cally conferred a strong selective advantage orbecause they are in linkage disequilibriumwith advantageous alleles. Although theincreased incidence of type 1 diabetes couldbe attributed to exposure to a novel environ-mental agent, such as a virus, given the lethal-ity of this autoimmune disease, it is moreprobable that the increase is a consequence of the current lack of infection, which fore-stalled the onset of diabetes in the past. Inrecent years, the following proposition hasbeen gaining ascendancy: that, over the past

© 2005 Nature Publishing Group
P E R S P E C T I V E S
is endemic become infected in early childhoodand might remain infected for life, owing tocontinuing susceptibility to infection andlongevity of adult worms10.
Schistosomes are adapted to, and mightpromote genetic change in, both their snail andhuman hosts. In snails, evidence for this is pro-vided by the observation that snail lines canbe selected for resistance and susceptibilityto specific parasite strains, with specificitybeing shown by resistance to the parasitestrain used for selection and not to a novelstrain11. Schistosomes use a wide range ofstrategies to evade the host immune response:for example, they use molecular mimicry toevade the immune defences of the snail host12.Schistosome-derived immunomodulatingneuropeptides seem to be equally effective inboth humans and snails. These might aidimmune evasion by the parasite, as their releasefrom parasites at stages of the life cycle thatoccur in both mammals and snails inhibits thelocomotory activity of immune cells from bothhosts13. In humans, a testament to the abilityof these parasites to avoid immune destruc-tion is provided by the observation that indi-vidual schistosomes live, fully exposed to theimmune system, in the bloodstream for up to40 years14. Schistosomes have an impressivearray of immune evasion and repair mecha-nisms, which include the ability to coatthemselves in host antigen, to enzymaticallycleave antibodies, and to rapidly replace theirsurface with unique double membranes,using pre-formed membrane vesicles15–18.
century, in the developed world, changes in economic and social conditions, as well asa concomitant reduction in exposure toinfectious agents (including helminths), havealtered the balance between our immune sys-tem and infections of historical importanceand that this is responsible for the observedincrease in autoimmunity. This is paralleled byobservations in the field of allergy, in which ithas been called the ‘hygiene hypothesis’4. Thisdoes not preclude the possibility that someautoimmune conditions are precipitated byexposure to infectious agents5, but there arenow many examples of both spontaneousand experimental models of human auto-immune diseases in which infection with a given agent inhibits the onset of auto-immune pathology (TABLE 1). There are manyways in which such infections might beexpected to impact on autoimmunity, and it isimportant to consider that an ideal responseto a pathogen would be one that does notinflict collateral damage on host tissue. Thisraises the issue of immunomodulation, whichwould be advantageous to both the micro-organism and the host. Insights into immuno-regulatory processes and how they mightregulate autoimmunity are provided bydetailed examination of the life cycles of theinfectious agents.
Helminths are old adversaries; helminthlarvae have been detected in samples of fos-silized animal faeces taken from archaeologicalsites of the Lower and Middle Pleistocene eras6.Schistosomes are members of the class trema-toda. Ancestral trematodes were probablysingle-host parasites of ancient marine mol-luscs, which with the emergence of predatoryvertebrates, developed complex life cyclesinvolving two or more different host speciesand both sexual and asexual reproduction.The parasites successfully diversified andexpanded, accompanying the molluscs as theyevolved into terrestrial and freshwater forms;consequently, all classes of vertebrate arenow host to many species of trematode, allof which still have intermediate hosts thatare snails. The ancestors of the schistosomesthat inhabit humans now were probably pre-sent in our ancestors before the evolution ofhumans as a species7. So, humans co-evolvedwith these schistosomes, as also occurred forother common parasitic worms, but the com-plexity and medico-biological implications ofthis co-evolution are not yet understood8.However, it has been shown that infectionwith schistosomes or exposure to schistosome-derived antigens prevents a range of auto-immune disorders in experimental animalmodels, including type 1 diabetes in non-obese diabetic (NOD) mice, experimental
allergic encephalomyelitis (EAE) (an animalmodel of multiple sclerosis) and Graves’ dis-ease (TABLE 1). We consider that, by examininghow a helminth such as Schistosoma mansonimight have evolutionarily influenced the hostimmune system9, we can gain insight intothe mechanisms that enable autoimmunityto develop in the absence of infection.
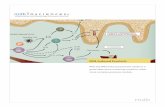
Schistosome–host interactionsSchistosome survival in the host. A diagram-matic representation of the life cycle ofS. mansoni is shown in FIG. 1. At present, intropical and subtropical areas, ∼200 millionpeople are suffering from chronic schisto-somiasis (BOX 1). Infection of humans occurswhen free-living, freshwater schistosome lar-vae, known as cercariae, come into contactwith and penetrate intact human skin. In theskin, the cercariae transform into a schisto-somular stage; these parasites then migratein the blood to the lungs and the liver. Aftermigration, the schistosomulum matures into amale or female adult worm. Schistosomes donot multiply in humans, but adult wormsmate in the blood vessels around the gut orbladder, depending on the species. Each femaleworm produces hundreds of eggs each day,and these need to reach fresh water to releasefree-swimming larvae (known as miracidia),which actively seek and penetrate the interme-diate aquatic snail host. Asexual reproductionin the snail produces thousands of infectivecercariae, which can then infect humans.People living in areas in which schistosomiasis
NATURE REVIEWS | IMMUNOLOGY VOLUME 5 | MAY 2005 | 421
Table 1 | Infectious agents, or their products, that prevent autoimmunity
Agent or product Autoimmune disease References
Schistosoma mansoni Type 1 diabetes 67Experimental allergic encephalomyelitis 68Graves’ thyroiditis 69
Schistosoma mansoni eggs Type 1 diabetes 67,71Experimental allergic encephalomyelitis 70Experimental colitis 72
Soluble Schistosoma mansoni Type 1 diabetes 32egg and worm products
Hymenolepis diminuta Experimental colitis 82
Acanthocheilonema viteae Collagen-induced arthritis 79secreted product (ES-62)
Trichuris suis Crohn’s disease 65,66
Heligmosomoides polygyrus Inflammatory bowel disease 80
Trichinella spiralis Experimental colitis 81
Trypanosoma brucei Collagen-induced arthritis 73
Mycobacterium bovis Type 1 diabetes 74Experimental allergic encephalomyelitis 75
Mycobacterium avium Type 1 diabetes 76
Streptococcus sanguinis* Collagen-induced arthritis 77
Bordetella pertussis Experimental allergic encephalomyelitis 78
*Previously known as Streptococcus sanguis.

© 2005 Nature Publishing Group
422 | MAY 2005 | VOLUME 5 www.nature.com/reviews/immunol
P E R S P E C T I V E S
antigens, including concomitant infections. Inthis way, it also has the potential to influenceautoimmunity.
Immune response to schistosomes. The initialimmune response after an individual isinfected is difficult to define, because childrengradually acquire more adult worms through-out early childhood. However, this responsehas been studied in mice infected with S. mansoni (FIG. 2). In this model of humaninfection, a T
H1-cell response to the parasite
develops during the first 5 weeks of infec-tion. However, as the infection progresses,and the worms mate and produce eggs, aT
H2-cell response emerges, and the T
H1- cell
response wanes27. By the time the immuneresponse is assessed in infected humans,there is a strongly polarised T
H2 response,
with increased numbers of eosinophils,basophils and mast cells, and high circulat-ing levels of IgE. There is evidence to indi-cate that helminths also downmodulate thisT
H2 response, because after clearance of the
parasite from humans treated with drugs,the in vitro parasite-specific T
H2-cell response
of peripheral-blood mononuclear cells isfurther increased28. Interleukin-10 (IL-10)-secreting cells have been found in increasednumbers in the peripheral blood of helminth-infected patients, and inclusion of IL-10-specific antibodies in in vitro cultures ofperipheral-blood mononuclear cells hasbeen shown to increase the cytokine responseto parasite-derived antigens29. The produc-tion of regulatory cytokines and T cells istherefore also a feature of chronic helminthinfections.
The early TH1-cell response that has been
noted after the initial infection in micemight be important for the parasite. Whenthe parasite first enters the host, prostaglan-din D
2(PGD
2) that is produced by the skin
stage of S. mansoni inhibits the migration of
japonicum26 are now revealing more parasitecomponents that are characteristic of all meta-zoans, including humans, implying that thereare many direct interactions between the para-site and the physiological and immunologicalprocesses of its human host. This ability tosubvert the host immune response has farreaching consequences and has been shown toinfluence the immune response to exogenous
Schistosomes not only use immune-evasion tactics but also subvert and utilize thehost immune response to facilitate their owndevelopment and transmission. To movefrom a human host to a snail host, S. mansonieggs must first translocate from the portalcapillaries to the gut lumen. This processdepends on host immune responses, as itdoes not occur in mice that are immuno-suppressed19 or in mice that have been toler-ized to S. mansoni eggs20. Egg excretion is alsogreatly reduced in HIV-infected individuals,in a manner that is proportional to the numberof circulating CD4+ T cells21. The productionof eggs by female worms has been suggested todepend on host tumour-necrosis factor(TNF)22. S. mansoni worms might also use hosttransforming growth factor-β (TGF-β) as aregulator of their growth and development23.A type I receptor serine/threonine kinase(RK1; also known as SmRK1) has been iden-tified on the surface tegument of S. mansoniworms24, and it can be activated by humanTGF-β and signals through the schistosomemolecule Smad2. The recently published trans-criptomes of S. mansoni 25 and Schistosoma
Snailintermediate host
Eggs(~140 µm)
Fresh water
Cercariae(~800 µm)
Host-derived mediators(TGF-β and TNF?)
Receptors for host-derived mediators
Adult male wormAdultfemaleworm
Liver
Portal vein
Intestine
Miracidia(~180 µm)
Figure 1 | Schistosoma mansoni life cycle. Infection of humans with Schistosoma mansoni is initiatedby cercariae, which burrow into the skin, transform into schistosomula, enter the vasculature and migrateto the portal system, where they mature into adult worms. S. mansoni adult worms mate in the bloodvessels. The eggs, which have tough shells, are released by female parasites within the vasculature; theythen cross the endothelium and basement membrane of the vein and traverse the intervening tissue,basement membrane and epithelium of the intestine (S. mansoni and Schistosoma japonicum eggs) orbladder (Schistosoma haematobium eggs) en route to the exterior. The passage of eggs to the outsidedepends on the host immune response. After they have reached fresh water, the eggs release a free-swimming larva (known as the miracidium), which actively seeks and penetrates its intermediate snail host. Asexual reproduction in the snail produces thousands of infective cercariae, which can then infecthumans. TGF-β, transforming growth factor-β; TNF, tumour-necrosis factor.
Box 1 | Human schistosomiasis
Three parasite species — Schistosoma mansoni, Schistosoma japonicum and Schistosomahaematobium — cause most of the human cases of schistosomiasis. After penetrating human skin,the parasites undergo complex transformations while migrating to their preferred intravenoussites, which they reach as mature worms 3–6 weeks later. Most severe morbidity is associated with chronic schistosome-egg-specific inflammation in the tissues of the gut (for infection with S. mansoni or S. japonicum) or the urogenital tract (for infection with S. haematobium). S. mansoniand S. japonicum eggs also become trapped in the liver, where after many years of infection,chronic T helper 2 (T
H2)-cell-mediated granulomatous responses can lead to gross periportal liver
fibrosis and life-threatening hepato-splenic schistosomiasis. Effective treatment, using the drugpraziquantel, has been available for 25 years, but the growth of human populations in high-riskareas, as well as the difficulties of delivering mass treatments and the high probability of rapid re-infection after treatment, have thwarted efforts to control the number of human infectionsworldwide. The S. mansoni-infected mouse has proved an invaluable model for the study of manyaspects of granulomatous inflammation, fibrosis and T
H1-cell versus T
H2-cell regulation.

© 2005 Nature Publishing Group
P E R S P E C T I V E S
schistosome infection46. Schistosome-specificphosphatidylserine activates TLR2 at the cellsurface of DCs, generating mature DCs withthe ability to promote the development ofIL-10-secreting regulatory T cells48. RegulatoryT cells induced by helminths have the potentialto suppress the T
H1-cell response to helminth-
derived antigens, thereby ensuring TH
2-cellpolarization49. The induction of regulatory T cells by these mechanisms might be animportant way of controlling over-vigorousimmune responses during the course ofchronic schistosome infection. Indeed, thereis evidence of regulation not only of T
H1- but
also of TH2-cell responses in individuals who
are infected with Schistosoma spp., and infectedindividuals have been shown to have reducedallergic responses50. This is associated withdiminished IL-5 production and increased IL-10 production by peripheral-blood mono-nuclear cells in response to antigens derivedfrom the house dust mite Dermatophagoides
Langerhans cells to the draining lymph nodes,thereby delaying the immune response30.Mice that are deficient in DP1, a receptor forPGD
2, do not show this inhibited migration
of skin dendritic cells (DCs), and cells in thedraining lymph node produce considerablyless interferon-γ (IFN-γ) and IL-10, butequal amounts of IL-4, leading to a moreT
H2-cytokine-biased response to the para-
site31. This is associated with a reduced num-ber of developing adult worms and decreasedimmune responses to parasite eggs, implyingthat this initial T
H1-cell response favours
infection. By studying mice and by examiningcell populations in vitro, it has been possibleto dissect key immunological interactionsthat influence the outcome of the immuneresponse to parasite infection.
Glycosylated components of schisto-some eggs have been shown to affect theproduction of cytokines by DCs32, and DCsstimulated by egg-derived antigens in vitrocan promote T
H2-cell responses in vivo 33.
Indeed, schistosome eggs are such powerfulT
H2-cell-response-promoting adjuvants that
they can promote TH2-cell responses to unre-
lated antigens in vivo 34. The glycoconjugatesthat influence DC maturation35 and functionsuch that a T
H2-cell response is induced
include lacto-N-fucopentaose III (REF. 36),which binds the DC-specific C-type lectinDC-SIGN (DC-specific intercellular adhe-sion molecule 3 (ICAM3)-grabbing non-integrin)37, and glycoproteins that containα3-fucose and core β2-xylose epitopes38.Recent experiments have shown that lyso-phosphatidylserine from helminth eggs acti-vated Toll-like receptor 2 (TLR2) at the cellsurface of DCs and influenced their functionalmaturation such that they induced IL-10-secreting regulatory T cells39. A schistosome-egg glycoprotein has also been identifiedthat induces the release of IL-4 and IL-13from basophils, by non-specifically bindingand possibly crosslinking cell-surface IgE40.Natural killer T (NKT) cells are also activatedand stimulated to proliferate by glycolipidsthat are present in both eggs and worms32.NKT cells, eosinophils and mast cells are there-fore all potential sources of IL-4; however, theabsence of these cell types does not preventthe establishment of T
H2-cell responses in
infected mice41,42. Egg glycoproteins can alsobind macrophage mannose receptors, and thismight be important in enabling alternativelyactivated macrophages to dampen inflamma-tory responses to schistosome eggs, therebypreventing organ damage. These macro-phages require IL-4 for their development,and mice that have macrophages deficientin the IL-4 receptor α-chain are extremely
susceptible to acute morbidity after infectionwith S. mansoni. This morbidity occurs con-comitantly with increased levels of T
H1-cell
cytokines43.In addition to a possible role in the matu-
ration of worms, TGF-β might also dampenthe host immune response. However, it is notclear which cells in the host produce thiscytokine or whether the worms themselveshave a role in the activation of latent TGF-β.By contrast, the immunoregulatory cytokineIL-10 is generated by cells of both the innateand adaptive immune systems in response toegg- and worm-derived antigens. Sources ofIL-10 include DCs, B cells and CD4+CD25+
regulatory T cells44–47. The production of IL-10by DCs and B cells would also facilitate thedevelopment of regulatory T cells. Recentstudies have highlighted the importance ofthe cooperative interaction between innateand adaptive sources of IL-10 in the genera-tion of a hyporesponsive state during chronic
NATURE REVIEWS | IMMUNOLOGY VOLUME 5 | MAY 2005 | 423
Imm
une
resp
onse
TH1cells
TH2 cells
IL-6TGF-βArginase
IL-4IL-5IL-13
IL-10?TGF-β?
IL-10
TNFIFN-γ
NO
IL-10IL-4
IL-10?TGF-β?
RegulatoryT cells
RegulatoryT cell
3 6 9 120
Cercariae Schistosomes Adult worms Eggs
B cellTH2 cellTH1 cellMacrophage Alternativelyactivatedmacrophage
Dendritic cellNKT cell
Time after infection (weeks)
Figure 2 | Induction of T-helper-1- and T-helper-2-cell responses and development of regulatory T cells after infection with Schistosoma mansoni. After infection with Schistosoma mansoni, the initialimmune response that develops is a T helper 1 (TH1)-cell response. As the worm develops and eggs aredeposited, natural killer T (NKT) cells are activated, dendritic cells produce more interleukin-10 (IL-10) andless IL-12, and a TH2-cell response develops. In addition, both B1 and B2 cells produce IL-10, in responseto egg-derived antigens and worm-derived antigens, respectively. Furthermore, populations of alternativelyactivated macrophages and regulatory T cells also develop. IFN-γ, interferon-γ; NO, nitric oxide; TGF-β, transforming growth factor-β; TNF, tumour-necrosis factor.

© 2005 Nature Publishing Group
424 | MAY 2005 | VOLUME 5 www.nature.com/reviews/immunol
P E R S P E C T I V E S
(TABLE 1). Protection from the ravages of pul-monary tuberculosis is associated with theproduction of cytokines such as TNF, IFN-γand IL-12. It might be predicted, therefore,that there would have been strong selectivepressure on human populations for individu-als able to mount a robust pro-inflammatoryresponse. This type of response is also onethat is associated with autoimmune diseasessuch as type 1 diabetes or rheumatoid arthritis.Although mycobacterial infections are associ-ated with T
H1-cell responses, it has recently
been shown that mycobacteria elicit IL-10 pro-duction by host cells. Indeed, the ability toinduce IL-10 rapidly might be a virulence fac-tor of mycobacteria, as this dampens down thepro-inflammatory response57. The myco-bacterial cell-wall component mannosylatedlipoarabinomannan targets DC-SIGN at thecell surface of DCs and induces IL-10 pro-duction58. This production of IL-10 mightcontribute to the development of regulatoryT-cell populations and inhibit inflammatoryresponses not only to the pathogen but alsoto self-antigens (FIG. 3).
Perturbing the balanceWhereas immunoparasitologists might talkabout a balance between the parasite and thehost, and between an immune response andimmune regulation, those concerned withevolution might discuss the arms race betweenthe host and the pathogen or the ‘Red Queenhypothesis’59 (BOX 2). This states that, from thepoint of view of the host, the host is runningto a stand still on an evolutionary treadmill dri-ven by parasites. This idea could be relevant tothe sudden disappearance of parasitic worms(and other infectious organisms) from a pro-portion of the human population, therebychanging a previously constant part of ourimmunomodulating internal environment.What happens if the treadmill suddenly stopsor if one of the teams in a tug of war lets go ofthe rope? Relationships between host and guestspecies range from the virulent to the benign,from mutualism to symbiosis. Although being
autoimmune pathology through a varietyof mechanisms (FIG. 3). First, worm-derivedantigens, through their ability to activate NKTcells and induce IL-10 production by B cells,have the potential to inhibit type 1 diabetesand possibly other autoimmune pathologies,such as EAE and arthritis, which are influ-enced by NKT-cell activity55. Interactions ofegg-derived antigens with DCs, together withthe induction of IL-10, could polarize theimmune response to a T
H2-cell response and
induce regulatory T-cell populations. In thecontext of T
H1-cell mediated autoimmune
diseases, a skewing of the immune responsetowards a T
H2-cell response would prevent
disease onset, as would the increased activ-ity of regulatory T cells. T
H2-cell-mediated
autoimmune pathologies would then becontrolled by IL-10 and TGF-β. However,there are additional mechanisms by whichautoimmune pathologies could be regulated.For example, adult worms have been shownto secrete opioids and opioid neuropeptides,such as pro-opiomelanocortin-derived pep-tides and enkephalin, which might modulatethe immune response56. Alternatively acti-vated macrophages are also induced duringinfection with S. mansoni, and these have thepotential to inhibit inflammatory responses43.
Although this article focuses on the effectsof infection with S. mansoni on autoimmunity,other helminths and microorganisms havebeen shown to inhibit self-reactivity (TABLE 1).In the past, individuals would have commonlybeen infected or exposed to more than one ofthese agents that give rise to chronic infec-tions, and they would have been selected onthe basis of their ability to survive such infec-tions. For example, similar to schistosomes,it has been estimated that mycobacteria founda niche in humans 10,000–20,000 years ago;therefore, humans and mycobacteria havehad a considerable period to engage in mutualco-adaptation and selection. Indeed, myco-bacterial infections and mycobacteria-derivedantigens also prevent the onset of diabetesand EAE in mouse models of autoimmunity
pteronyssinus 51. The observation that a rangeof infectious agents have evolved strategies toinduce IL-10 production, or to encode theirown surrogate IL-10, indicates that it hasproven to be an important mechanism forsuppressing host immune responses, even if itis used only in the short term to establish alatent infection (in the case of some viruses)while limiting pathology in the host52–54.
Infection and autoimmunityFrom the studies that we have discussed here,it is clear that organisms such as Schistosomaspp., which have long been part of the humanenvironment, influence the immune responsein infected individuals and have the potentialto influence autoimmune responses. Experi-mental studies in rodents have shown thatinfection with S. mansoni prevents the onsetof diabetes, as well as of other autoimmunedisorders (TABLE 1). Molecules produced by S. mansoni at different stages of its life cyclein the mammalian host have the potentialto inhibit both T
H1- and T
H2-cell-mediated
Allergy
TH1 cell
IL-10TGF-β
IL-10TGF-β
EAEColitisDiabetesArthritis
B cell
Dendriticcell
TH0 cell Regulatory T cell
Helminth-derivedantigens
NKT cell
TH2 cell
IL-10
IL-10
IL-4
Figure 3 | Mechanisms by which helminthscould impact on autoimmunity and allergy.Helminth-derived antigens influence thedevelopment of T-cell responses throughinteractions with dendritic cells (DCs), naturalkiller T (NKT) cells and B cells. Interleukin-10 (IL-10) production by B cells and DCs could induceregulatory T-cell activity and thereby prevent thedevelopment of autoaggressive T helper 1 (TH1)-cellresponses, skewing the response towards a non-autoimmune-disease-promoting TH2-cell responseinstead. In addition, regulatory T cells might preventthe development of a range of autoimmunedisorders, as well as allergy, through the productionof IL-10 and transforming growth factor-β (TGF-β).EAE, experimental allergic encephalomyelitis.
Box 2 | The ‘Red Queen hypothesis’
The Red Queen hypothesis was originally proposed by Leigh Van Valen59 as an explanation for the evolution of sex. During gamete formation, at meiosis, there is the possibility ofreassortment and exchange of genetic information between chromosomes, so together withmutation, this contributes to the development of genetically diverse individuals. Such geneticdiversity enables the host organism to combat infection. As the host organism evolves in thisway, so too does the infectious agent. It is, in essence, a description of the events that occurduring the co-evolution of a host and its parasite, in which each is attempting to evade thedefences of the other. It is known as the Red Queen hypothesis because it is taken from LewisCarroll’s Through the Looking Glass (1872), when the Red Queen tells Alice,“[I]t takes all therunning you can do, to keep in the same place.”

© 2005 Nature Publishing Group
P E R S P E C T I V E S
22. Amiri, P. et al. Tumour necrosis factor α restoresgranulomas and induces parasite egg-laying inschistosome-infected SCID mice. Nature 356, 565–566(1992).
23. Beall, M. J. & Pearce, E. J. Human transforming growthfactor-β activates a receptor serine/threonine kinase fromthe intravascular parasite Schistosoma mansoni. J. Biol.Chem. 276, 31613–31619 (2001).
24. Davies, S. J., Shoemaker, C. B. & Pearce, E. J. A divergent member of the transforming growth factor receptor family from Schistosoma mansoni is expressed on the parasite surface membrane. J. Biol. Chem. 273, 11234–11240 (1998).
25. Verjovski-Almeida, S. et al. Transcriptome analysis of the acoelomate human parasite Schistosoma mansoni.Nature Genet. 35, 148–157 (2003).
26. Hu, W. et al. Evolutionary and biomedical implications of a Schistosoma japonicum complementary DNAresource. Nature Genet. 35, 139–147 (2003).
27. Grzych, J. M. et al. Egg deposition is the major stimulusfor the production of TH2 cytokines in murineschistosomiasis mansoni. J. Immunol. 146, 1322–1327(1991).
28. Grogan, J. L., Kremsner, P. G., Deelder, A. M. &Yazdanbakhsh, M. Antigen-specific proliferation and interferon-γ and interleukin-5 production are down-regulated during Schistosoma haematobium infection. J. Infect. Dis. 177, 1433–1437 (1998).
29. Grogan, J. L., Kremsner, P. G., Deelder, A. M. &Yazdanbakhsh, M. The effect of anti-IL-10 onproliferation and cytokine production in humanschistosomiasis: fresh versus cryopreserved cells.Parasite Immunol. 20, 345–349 (1998).
30. Angeli, V. et al. Role of the parasite-derivedprostaglandin D2 in the inhibition of epidermalLangerhans cell migration during schistosomiasisinfection. J. Exp. Med. 193, 1135–1147 (2001).
31. Herve, M. et al. Pivotal roles of the parasite PGD2 synthaseand of the host D prostanoid receptor 1 in schistosomeimmune evasion. Eur. J. Immunol. 33, 2764–2772 (2003).
32. Zaccone, P. et al. Schistosoma mansoni antigensmodulate the activity of the innate immune response and prevent onset of type 1 diabetes. Eur. J. Immunol.33, 1439–1449 (2003).
33. MacDonald, A. S., Straw, A. D., Bauman, B. & Pearce, E. J. CD8– dendritic cell activation status plays an integral role in influencing TH2 responsedevelopment. J. Immunol. 167, 1982–1988 (2001).
34. Kullberg, M. C., Pearce, E. J., Hieny, S. E., Sher, A. &Berzofsky, J. A. Infection with Schistosoma mansonialters TH1/TH2 cytokine responses to a non-parasiteantigen. J. Immunol. 148, 3264–3270 (1992).
35. Thomas, P. G. et al. Maturation of dendritic cell 2phenotype by a helminth glycan uses a Toll-like receptor 4-dependent mechanism. J. Immunol. 171, 5837–5841(2003).
36. Okano, M., Satoskar, A. R., Nishizaki, K. & Harn, D. A.Lacto-N-fucopentaose III found on Schistosoma mansoniegg antigens functions as adjuvant for proteins by inducingTH2-type response. J. Immunol. 167, 442–450 (2001).
37. van Die, I. et al. The dendritic cell-specific C-type lectinDC-SIGN is a receptor for Schistosoma mansoni eggantigens and recognizes the glycan antigen Lewis X.Glycobiology 13, 471–478 (2003).
38. Faveeuw, C. et al. Schistosome N-glycans containingcore α3-fucose and core β2-xylose epitopes are stronginducers of TH2 responses in mice. Eur. J. Immunol.33, 1271–1281 (2003).
39. Kane, C. M. et al. Helminth antigens modulate TLR-initiated dendritic cell activation. J. Immunol.173, 7454–7461 (2004).
40. Schramm, G. et al. Molecular characterization of aninterleukin-4-inducing factor from Schistosoma mansonieggs. J. Biol. Chem. 278, 18384–18392 (2003).
41. Sabin, E. A., Kopf, M. A. & Pearce, E. J. Schistosomamansoni egg-induced early IL-4 production is dependentupon IL-5 and eosinophils. J. Exp. Med. 184, 1871–1878(1996).
42. Brown, D. R. et al. β2-Microglobulin-dependent NK1.1+
T cells are not essential for T helper cell 2 immuneresponses. J. Exp. Med. 184, 1295–1304 (1996).
43. Herbert, D. R. et al. Alternative macrophage activation is essential for survival during schistosomiasis anddownmodulates T helper 1 responses andimmunopathology. Immunity 20, 623–635 (2004).
44. Sher, A., Fiorentino, D., Caspar, P., Pearce, E. &Mosmann, T. Production of IL-10 by CD4+ T lymphocytescorrelates with down-regulation of TH1 cytokine synthesisin helminth infection. J. Immunol. 147, 2713–2716(1991).
detrimental to its hosts is a key element in thedefinition of a parasite, it has been suggestedthat there might be some contexts, particularlyoutside the natural ecological environment ofthe host, in which parasitized individuals havean advantage over uninfected individuals60.A particular immune response bias, a T
H1-cell
bias in the case of infections with intracellularpathogens or a T
H2-cell bias for infections
with helminths, could be advantageous for the host until the parasite that this responseprotects against is no longer present. However,if the checks and balances that prevent theimmune response from causing damage to thehost also depend on the parasite, the immuneresponse could become a liability. In this situa-tion, we are outside the realm of evolution andinto the realm of public health, where perhapsa few parasites are a good thing; if so, they areno longer parasitic but have become symbiotic.
In the context of autoimmunity, studiesin animal models show that infection withhelminths or with some bacteria prevent theonset of disease. In terms of the mechanism ofprevention of autoimmune disease, manyof the schistosome-induced host immuneresponses that are outlined here could have arole. Removal of these infections, and there-fore removal of exposure to the immuno-modulating agents they encode, could allowthe development of type 1 diabetes and otherautoimmune disorders, as well as allergy 61–64
(FIG. 3). In addition, the loss of our formerhelminth companions might exacerbateT
H1-cell-mediated autoimmunity through a
lack of TH2-cell-dependent counter-regulation.
It is on this basis that infection with liveworms has been advocated as a therapy forinflammatory bowel disease65,66. Owing to ourincreasing understanding of how these infec-tious agents harness, utilize and subvert theimmune response, it is probable that mol-ecules derived from parasites and bacteria willreplace live infection to prevent or controlpro-inflammatory pathological responses.
ConclusionOur immune system has been shaped overtime by exposure to infectious agents, some ofwhich harness the host immune response tofacilitate their own life cycles.A key element inthis co-evolution is the need to dampen downthe immune response such that host pathol-ogy is minimized. Here, we have consideredhow alterations in the balance between thehost and the parasite could enable pathologi-cal responses to emerge in the host. As themolecular mechanisms by which infectiousagents interface with the immune systembecome clearer, we anticipate that a range ofparasite-derived biomodulators that can elicit
anti-inflammatory responses will be identi-fied, and these could be of considerabletherapeutic value.
David W. Dunne and Anne Cooke are at theDepartment of Pathology, University of
Cambridge, Cambridge, UK.Correspondence to A.C.
e-mail: [email protected]
doi:10.1038/nri1601
1. Aaltonen, J. & Bjorses, P. Cloning of the APECED gene provides new insight into human autoimmunity.Ann. Med. 31, 111–116 (1999).
2. Hyttinen, V., Kaprio, J., Kinnunen, L., Koskenvuo, M. &Tuomilehto, J. Genetic liability of type 1 diabetes and the onset age among 22,650 young Finnish twin pairs: a nationwide follow-up study. Diabetes 52, 1052–1055(2003).
3. Banting, F. G. & Best, C. H. The internal secretion of thepancreas. J. Lab. Clin. Med. 7, 465–480 (1922).
4. Strachan, D. P. Hay fever, hygiene, and household size.BMJ 299, 1259–1260 (1989).
5. Jaeckel, E., Manns, M. & von Herrath, M. Viruses anddiabetes. Ann. NY Acad. Sci. 958, 7–25 (2002).
6. Goncalves, M. L., Araujo, A. & Ferreira, L. F. Humanintestinal parasites in the past: new findings and a review. Mem. Inst. Oswaldo Cruz 98, 103–118 (2003).
7. Basch, P. F. Schistosomes: Development, Reproduction,and Host Relations (Oxford Univ. Press, New York, 1991).
8. Woolhouse, M. E. J., Webster, J. P., Domingo, E.,Charlesworth, E. B. & Levin, B. R. Biological andbiomedical implications of the co-evolution of pathogensand their hosts. Nature Genet. 32, 569–577 (2002).
9. Pearce, E. J. & MacDonald, A. S. The immunobiology of schistosomiasis. Nature Rev. Immunol. 2, 499–511(2002).
10. Sturrock, R. F. in Human Schistosomiasis (eds Jordan, P.,Webbe, G. & Sturrock, R. F.) 1–32 (CAB International,Wallingford, 1993).
11. Webster, J. P. & Woolhouse, M. E. J. Heritability andstrain specificity in compatibility between snailintermediate hosts and their parasitic schistosomes.Evolution 52, 1627–1634 (1998).
12. Dissous, C., Torpier, G., Duvaux-Miret, O. & Capron, A.Structural homology of tropomyosins from the humantrematode Schistosoma mansoni and its intermediatehost Biomphalaria glabrata. Mol. Biochem. Parasitol.43, 245–255 (1990).
13. Duvaux-Miret, D., Stefano, G. B., Smith, E. M.,Mallozzi, L. A. & Capron A. Proopiomelanocortin-derived peptides as tools of immune evasion for thehuman trematode Schistosoma mansoni. Acta Biol.Hung. 43, 281–286 (1992).
14. Chabasse, D., Bertrand, G., Leroux, J. P., Gauthey, N. &Hocquet, P. Developmental bilharziasis caused bySchistosoma mansoni discovered 37 years afterinfestation. Bull. Soc. Pathol. Exot. Filiales 78, 643–647(1985) (in French).
15. Pearce, E. J. & Sher, A. Mechanisms of immune evasion inschistosomiasis. Contrib. Microbiol. Immunol. 8, 219–232(1987).
16. Damian, R. T. Molecular mimicry: parasite evasion and host defense. Curr. Top. Microbiol. Immunol.145, 101–115 (1989).
17. Maizels, R. M., Bundy, D. A., Selkirk, M. E., Smith, D. F. &Anderson, R. M. Immunological modulation and evasionby helminth parasites in human populations. Nature365, 797–805 (1993).
18. Carvalho-Queiroz, C. et al. Cross-reactivity ofSchistosoma mansoni cytosolic superoxide dismutase, a protective vaccine candidate, with host superoxidedismutase and identification of parasite-specific B epitopes. Infect. Immun. 72, 2635–2647 (2004).
19. Doenhoff, M. J. A role for granulomatous inflammation in the transmission of infectious disease: schistosomiasisand tuberculosis. Parasitology 115, S113–S125 (1977).
20. Fallon, P. G. & Dunne, D. W. Tolerization of mice toSchistosoma mansoni egg antigens causes elevatedtype 1 and diminished type 2 cytokine responses andincreased mortality in acute infection. J. Immunol.162, 4122–4132 (1999).
21. Karanja, D. M., Colley, D. G., Nahlen, B. L., Ouma, J. H. &Secor, W. E. Studies on schistosomiasis in westernKenya: I. Evidence for immune-facilitated excretion ofschistosome eggs from patients with Schistosomamansoni and human immunodeficiency virus coinfections.Am. J. Trop. Med. Hyg. 56, 515–521 (1997).
NATURE REVIEWS | IMMUNOLOGY VOLUME 5 | MAY 2005 | 425

© 2005 Nature Publishing Group
426 | MAY 2005 | VOLUME 5 www.nature.com/reviews/immunol
P E R S P E C T I V E S
76. Bras, A. & Aguas, A. P. Diabetes-prone NOD mice areresistant to Mycobacterium avium and the infectionprevents autoimmune disease. Immunology 89, 20–25(1996).
77. Costalonga, M., Hodges, J. S. & Herzberg, M. C.Streptococcus sanguis modulates type II collagen-induced arthritis in DBA/1J mice. J. Immunol.169, 2189–2195 (2002).
78. Lehmann, D. & Ben-Nun, A. Bacterial agents protectagainst autoimmune disease. I. Mice pre-exposed toBordetella pertussis or Mycobacterium tuberculosis arehighly refractory to induction of experimental autoimmuneencephalomyelitis. J. Autoimmun. 5, 675–690 (1992).
79. McInnes, I. B. et al. A novel therapeutic approachtargeting articular inflammation using the filarialnematode-derived phosphorylcholine-containingglycoprotein ES-62. J. Immunol. 171, 2127–2133 (2003).
80. Elliott, D. E. et al. Heligmosomoides polygyrus inhibitsestablished colitis in IL-10-deficient mice. Eur. J. Immunol.34, 2690–2698 (2004).
81. Khan, W. I. et al. Intestinal nematode infectionameliorates experimental colitis in mice. Infect. Immun.70, 5931–5937 (2002).
82. Reardon, C., Sanchez, A., Hogaboam, C. M. & McKay, D. M. Tapeworm infection reduces epithelial iontransport abnormalities in murine dextran sulfate sodium-induced colitis. Infect. Immun. 69, 4417–4423 (2001).
AcknowledgementsWe are grateful to the Wellcome Trust (United Kingdom) andDiabetes UK, who have supported our research into the abilityof infections to modulate autoimmunity. We thank J. Connor, P. Zaccone, H. Cronin, J. Cooke, S. Efstathiou and T. Raine fordiscussions and for help in the preparation of this manuscript.
Competing interests statementThe authors declare no competing financial interests.
Online links
DATABASESThe following terms in this article are linked online to:Entrez Gene:http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=geneIFN-γ | IL-4 | IL-10 | IL-12 | TGF-β | TNF Infectious Disease Information:http://www.cdc.gov/ncidod/diseases/index.htmschistosomiasis OMIM:http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=OMIMGraves’ disease | inflammatory bowel disease | multiplesclerosis | systemic lupus erythematosus | type 1 diabetes
FURTHER INFORMATIONDavid Dunne’s homepage:http://www.path.cam.ac.uk/pages/dunne/Anne Cooke’s homepage:http://www.path.cam.ac.uk/pages/cooke/Access to this interactive links box is free online.
61. Maizels, R. M. & Yazdenbakhsh, M. Immune regulation by helminth parasites: cellular and molecular mechanisms.Nature Rev. Immunol. 3, 733–744 (2003).
62. Cooke, A., Zaccone, P., Raine, T., Phillips, J. M. & Dunne, D. W. Infection andautoimmunity: are we winning the war, only to lose the peace? Trends Parasitol.20, 316–321 (2004).
63. Wilson, M. S. & Maizels, R. M. Regulation of allergy andautoimmunity in helminth infection. Clin. Rev. AllergyImmunol. 26, 35–50 (2004).
64. Elliott, D. E., Summers, R. W. & Weinstock, J. V.Helminths and the modulation of mucosalinflammation. Curr. Opin. Gastroenterol. 21, 51–58 (2005).
65. Summers, R. W. et al. Trichuris suis seems to be safe and possibly effective in the treatment of inflammatorybowel disease. Am. J. Gastroenterol. 98, 2034–2041(2003).
66. Summers, R. W., Elliott, D. E., Urban, J. F., Thompson, R. & Weinstock, J. V. Trichuris suis therapy in Crohn’s disease. Gut 54, 87–90 (2005).
67. Cooke, A. et al. Infection with Schistosoma mansoniprevents insulin dependent diabetes mellitus in non-obese diabetic mice. Parasite Immunol. 21, 169–176(1999).
68. La Flamme, A. C., Ruddenklau, K. & Backstrom, B. T.Schistosomiasis decreases central nervous systeminflammation and alters the progression of experimentalautoimmune encephalomyelitis. Infect. Immun.71, 4996–5004 (2003).
69. Nagayama, Y., Watanabe, K., Niwa, M., McLachlan, S. M. & Rapoport, B. Schistosomamansoni and α-galactosylceramide: prophylactic effectof TH1 immune suppression in a mouse model ofGraves’ hyperthyroidism. J. Immunol. 173, 2167–2173(2004).
70. Sewell, D. et al. Immunomodulation of experimentalautoimmune encephalomyelitis by helminth ovaimmunization. Int. Immunol. 15, 59–69 (2003).
71. El-Wakil, H. S. et al. Effect of Schistosoma mansoniegg deposition on multiple low doses streptozotocininduced insulin dependent diabetes. J. Egypt Soc.Parasitol. 32, 987–1002 (2002).
72. Elliott, D. E. et al. Exposure to schistosome eggsprotects mice from TNBS-induced colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 284,G385–G391 (2003).
73. Mattsson, L., Larsson, P., Erlandsson-Harris, H.,Klareskog, L. & Harris, R. A. Parasite-mediated down-regulation of collagen-induced arthritis (CIA) in DA rats.Clin. Exp. Immunol. 122, 477–483 (2000).
74. Baxter, A. G. et al. Mycobacteria precipitate an SLE-likesyndrome in diabetes-prone NOD mice. Immunology83, 227–231 (1994).
75. Sewell, D. L. et al. Infection with Mycobacterium bovisBCG diverts traffic of myelin oligodendroglial glycoproteinautoantigen-specific T cells away from the centralnervous system and ameliorates experimentalautoimmune encephalomyelitis. Clin. Diagn. Lab.Immunol. 10, 564–572 (2003).
45. Palanivel, V. et al. B-cell outgrowth and ligand-specificproduction of IL-10 correlate with TH2 dominance incertain parasitic diseases. Exp. Parasitol. 84, 168–177(1996).
46. Hesse, M. et al. The pathogenesis of schistosomiasis iscontrolled by cooperating IL-10-producing innate effectorand regulatory T cells. J. Immunol. 172, 3157–3166(2004).
47. Mangan, N. E. et al. Helminth infection protects micefrom anaphylaxis via IL-10-producing B cells. J. Immunol.173, 6346–6356 (2004).
48. van der Kleij, D. et al. A novel host–parasite lipid cross-talk. Schistosomal lyso-phosphatidylserine activates Toll-like receptor 2 and affects immune polarization. J. Biol. Chem. 277, 48122–48129 (2002).
49. McKee, A. S. & Pearce, E. J. CD25+CD4+ cellscontribute to TH2 polarization during helminth infectionby suppressing TH1 response development. J. Immunol.173, 1224–1231 (2004).
50. van den Biggelaar, A. H. et al. Decreased atopy inchildren infected with Schistosoma haematobium: a role for parasite-induced interleukin-10. Lancet356, 1723–1727 (2000).
51. Araujo, M. I. et al. Impaired T helper 2 response toaeroallergen in helminth-infected patients with asthma.J. Infect. Dis. 190, 1797–1803 (2004).
52. Redpath, S., Ghazal, P. & Gascoigne, N. R. Hijacking and exploitation of IL-10 by intracellular pathogens.Trends Microbiol. 9, 86–92 (2001).
53. Mahot, S., Sergeant, A., Drouet, E. & Gruffat, H. A novelfunction for the Epstein–Barr virus transcription factorEB1/Zta: induction of transcription of the hIL-10 gene. J. Gen. Virol. 84, 965–974 (2003).
54. Peacock, J. W. & Bost, K. L. Murine γ-herpesvirus-68-induced interleukin-10 increases viral burden, but limitsvirus-induced splenomegaly and leukocytosis.Immunology 104, 109–117 (2001).
55. Godfrey, D. I. & Kronenberg, M. Going both ways:immune regulation via CD1d-dependent NKT cells. J. Clin. Invest. 114, 1379–1388 (2004).
56. Pryor, S. C. & Elizee, R. Evidence of opiates and opioidneuropeptides and their immune effects in parasiticinvertebrates representing three different phyla:Schistosoma mansoni, Theromyzon tessulatum,Trichinella spiralis. Acta Biol. Hung. 51, 331–341 (2000).
57. Theus, S. A., Cave, M. D. & Eisenach, K. D. Intracellularmacrophage growth rates and cytokine profiles ofMycobacterium tuberculosis strains with differenttransmission dynamics. J. Infect. Dis. 191, 453–460(2005).
58. Koppel, E. A. et al. Identification of the mycobacterialcarbohydrate structure that binds the C-type lectins DC-SIGN, L-SIGN and SIGNR1. Immunobiology209, 117–127 (2004).
59. Van Valen, L. A new evolutionary law. Evol. Theory1, 1–30 (1973).
60. Thomas, F., Poulin, R., Guegan, J.-F., Michalakis, Y. &Renaud, F. Are there pros as well as cons to beingparasitized? Parasitol. Today 16, 533–536 (2000).