MCP-1 and MIP-1α are Most Efficient in Recruiting T Cells into the Skin In Vivo
Transcript of MCP-1 and MIP-1α are Most Efficient in Recruiting T Cells into the Skin In Vivo

MCP-1 and MIP-1α are Most Efficient in Recruiting T Cellsinto the Skin In Vivo
Rainer Kunstfeld, Sonja Lechleitner, Klaus Wolff, and Peter PetzelbauerDepartment of Dermatology, Division of General Dermatology, University of Vienna Medical School, Vienna, Austria
To analyze T cell recruitment by chemokines in vivo, weused SCID mice grafted with human skin onto theirbacks and human T cells into their peritoneal cavities.rh MIP-1α and rh MCP-1, as well as mouse MCP-1,attracted significant amounts of human T cells intohuman skin grafts, whereas effects of rh RANTES, rhSDF-1, or rh IP-10 were minimal. Human T cells werefound in a striking perivascular position exclusively atsites of human endothelium. None of the chemokinesrecruited human T cells across mouse endothelial barriers.
Postcapillary venules are the sites for white blood cellemigration from the blood stream into the tissue. Theyare in a unique position to control for both the specificityand the proportions of the inflammatory reaction. Themolecular basis for the recruitment of leukocytes through
the endothelial barrier is partially based on the selective expression ofadhesion molecules, which largely belong to the selectin or integrinfamily of proteins. Yet a large body of in vitro experiments suggests thatit also involves chemokines (Baggiolini et al, 1997; Bazan et al, 1997).
It was originally thought that chemokine-induced tissue inflamma-tion functions solely along a concentration gradient of the givenchemoattractant to achieve directionality of movement, suggesting thatchemokines might function independently from endothelial-leukocyteadhesion pathways. Employing in vitro migration assays using Boydenchambers, it was possible to identify chemokines that were chemotacticfor T cells (Roth et al, 1995a; Taub et al, 1995a). A second importantfunction, the induction of downstream activation of integrins, whichstrengthens the molecular basis of leukocyte–endothelial cell interactions(Carr et al, 1996; Weber et al, 1996; Campbell et al, 1998), hasbeen assigned to chemokines, suggesting that chemokines need thecollaboration with endothelial cells to execute this function. Employingin vitro flow chamber assays, it was possible to identify chemokines thattrigger this integrin-dependent pathway of adhesion leading to thearrest of rolling T cells (Campbell et al, 1998).
Because in vitro assays cannot mimic the complexity of chemokinefunction in T cell homing in vivo, we designed an animal model to,first, quantitate the ability of a given chemokine to induce T cellinflammation and, second, investigate the role of endothelial cells inchemokine-induced T cell inflammation. We selected a battery ofchemokines with reported T cell chemotactic abilities in vitro: C-C
Manuscript received April 22, 1998; revised July 23, 1998; accepted forpublication July 29, 1998.
Reprint requests to: Dr. Peter Petzelbauer, Department of Dermatology,University of Vienna Medical School, Waehringer Guertel 18-20, A-1090Vienna, Austria.
Abbreviations: DVP, deep vascular plexus; SVP, superficial vascular plexus.
0022-202X/98/$10.50 · Copyright © 1998 by The Society for Investigative Dermatology, Inc.
1040
For control purposes, chemokines were injected into theabdominal wall directly above the peritoneal cavity,where human T cells had been injected and were notsubjected to flow. At this site all tested chemokinesattracted human T cells to an equal extent. In summary,our experiments point towards an important role forMCP-1 and MIP-1α as T cell chemoattractants for skininflammatory conditions. Key words: chemokine/endothe-lium/inflammation/SCID mouse/skin graft. J Invest Dermatol111:1040–1044, 1998
chemokines MCP-1, MIP-1α, and RANTES (del Pozo et al, 1995;Roth et al, 1995b; Taub et al, 1995b; Weber et al, 1996), and the C-X-C chemokines SDF-1 and IP-10 (Taub et al, 1993; Bleul et al,1996). We took advantage of our Hu-SCID mouse model grafted withhuman skin and human T cells (Petzelbauer et al, 1996; Kunstfeld et al,1997), which has two phenotypically different vascular beds, the pre-existing mouse vessels and the human vessels grafted together with thehuman skin (Murray et al, 1994). This model allowed us to test forthe effects of a given chemokine on human T cell recruitment in anin vivo-like setting at sites of human as well as xenogeneic mouseendothelium.
MATERIALS AND METHODS
Reagents Anti-human CD3, CD4, CD8, CD45RA, and CD45RO (allmouse IgG1) were from Dako (Glostrup, Denmark) and anti-mouse CD31(clone 2H8, hamster IgG) was from Endogen (Woburn, MA). BiotinylatedUlex europeus agglutinin-1 (UEA-1) was from Vector Laboratories (Burlingame,CA). The chemokines rh MIP-1α, rh MCP-1, and rh RANTES were fromEndogen, and rh SDF-1, rh IP-10, and recombinant mouse MCP-1 were fromR&D Systems (Minneapolis, MN). Chemokines were reconstituted with sterilephosphate-buffered saline containing 0.5% bovine serum albumin as carrierprotein at a concentration of 200 ng per 50 µl. rh TNF-α was from GenzymeDiagnostics (Cambridge, MA).
Human skin and human T cell engraftment CB17 SCID mice werefrom Bomholtgård (Bomice, Denmark). Details of animal care have beendescribed previously (Petzelbauer et al, 1996). Normal human skin was obtainedfrom cadaveric donors (Department of Pathology, University of Vienna MedicalSchool). All experimental protocols were approved by the University of ViennaAnimal Care and Use Committee and the University of Vienna EthicalCommittee. Superficial portions of the human skin containing epidermis andpapillary dermis (superficial vascular plexus, SVP) as well as deep portions ofthe skin containing the reticular dermis (deep vascular plexus, DVP), wereharvested with a dermatome as described previously (Kunstfeld et al, 1997).The resulting sheets of SVP as well as of DVP skin had an approximate thicknessof 0.5–0.8 mm and were cut into pieces of 10 3 10 mm. One SVP and oneDVP sample were grafted onto the left and right back side of a mouse asdescribed previously (Kunstfeld et al, 1997). Fourteen days after skin engraftment,antiasialo GM1 (50 µg; Wako, Neuss, Germany) was injected intraperitoneallyinto SCID mice to remove mouse natural killer cells. Twenty-four hours later

VOL. 111, NO. 6 DECEMBER 1998 CHEMOKINES IN T CELL INFLAMMATION 1041
1.3 3 108 human peripheral blood mononuclear cells (PBMC) isolated frombuffy coats as described previously (Petzelbauer et al, 1996), were injectedintraperitoneally into each mouse.
Experimental protocol We used skin from 10 different donors for a totalof 140 mice. One hundred and twenty mice thereof received PBMC from 30different buffy coat donors. One buffy coat was needed to reconstitute one setof four mice resulting in 30 sets (30 3 4 mice). Within in one set, each of thefour mice had skin grafts from the same human skin donor and PBMC graftsfrom the same human buffy coat donor. In each set, one mouse was injectedwith 50 µl solvent control, the second mouse received 1000 U TNF dilutedin 50 µl solvent, and the remaining two mice received the indicated chemokines(200 ng per injection site diluted in 50 µl solvent). The injection sites in eachmouse were human SVP skin, human DVP skin, mouse back skin, and mousegluteal muscle, and, in selected experiments, the abdominal muscles directlyabove the peritoneal cavity. The remaining 20 mice did not receive PBMCgrafts (PBMC– mice). Chemokines injected into such PBMC– mice did notalter numbers of resident T cells and did not cause recruitment of mouse whiteblood cells at any site (data not shown). To control for endotoxin contamination,chemokines were boiled before injection, which resulted in a complete loss oftheir activity.
Figure 1. Time kinetics of T cell infiltrates following chemokineinjection. A cocktail of rh MIP-1a, MCP-1, and RANTES, 200 ng each, wasinjected into the human skin sites 5, 7, or 9 d following grafting of human Tcells into the peritoneal cavity. The skin specimens were harvested at indicatedtimes following chemokine injection and numbers of T cells were counted incryostat sections stained with an anti-CD3 monoclonal antibody as describedin Materials and Methods. SVP, superficial vascular plexus skin; DVP, deepvascular plexus skin. One of two experiments is shown.
Figure 2. MCP-1-induced T cell infiltrates are restricted to sites ofhuman endothelium. MCP-1 (left panel) was injected into human skin,mouse skin, or mouse muscle 7 d following T cell engraftment, skin specimenswere harvested 48 h following the intradermal MCP-1 injection, and numbersof T cells per high power field were enumerated; results of three independentexperiments are shown. TNF was used as a positive control (right panel).
Immunohistochemistry and statistical evaluation Mice were sacrificedand the respective tissues were divided into two halves, one half was preparedfor immunohistochemistry, the second half was directly snap frozen and storedin liquid nitrogen for mRNA isolation. Five micrometer cryostat sections werecut and incubated with first step reagents, for T cell typing with anti-humanCD3, CD4, CD8, CD45RA, and CD45RO, for visualization of mouse vesselswith anti-mouse CD31, and for human vessels with biotinylated UEA-1.Monoclonal antibody binding was visualized as described previously (Murrayet al, 1994; Kunstfeld et al, 1997). Double staining was performed as describedpreviously (Petzelbauer et al, 1993) using the APAAP system (Dako) for bluestaining and the avidin-biotin-peroxidase system (Vector) for red staining.
Absolute numbers of human T cells were counted by two independentobservers in each specimen and expressed as cells per high power field (coveringan area 0.3 mm2). In order to normalize for interexperimental differencesbetween distinct sets of four mice, we calculated the relative increase in numbersof infiltrating T cells in response to the indicated chemokine as compared withits respective saline control, which was then expressed as ‘‘x-fold’’ increase.
Analysis of cytokine mRNA expression Tissues (100 mg per sample)were snap frozen, homogenized, and mRNA directly isolated by using magneticoligo (dT25) beads. Ten microliters of each mRNA sample was reversetranscribed and 5 µl cDNA was amplified as described previously (Kunstfeldet al, 1997). Primers for human GAPDH, IL-2, IL-4, IFN-γ, or mouse GAPDHwere from Clontech (Palo Alto, CA). PCR products were analyzed in ethidiumbromide stained agarose gels (1.5%) and identified by their size.
RESULTS
The Hu-SCID mouse model allows us to examine human T cellrecruitment upon chemokine injection We have previouslyreported phenotypic characteristics of engrafted human skin and kineticsof T cell reconstitution within the SCID mouse circulation (Murrayet al, 1994; Petzelbauer et al, 1996; Kunstfeld et al, 1997). For reasonsof availability, the human skin and human T cells used in theseexperiments were from different donors. In such an allogeneic system,human T cells will eventually migrate into human grafts, even withoutchemokine injection (Murray et al, 1994). In a first series of experimentswe therefore defined a window, where reconstitution of human T cellswithin the mouse circulation was sufficient to investigate chemokine-induced T cell inflammation without having T cell infiltrates due to
Figure 3. Chemokine differences in attracting human T cells. Chemokineswere injected at day 7 following T cell engraftment, human skin was harvested48 h later. Results are shown as the x-fold increase in numbers of T cells inchemokine-injected skin sites compared with its individual solvent control.Logarithmic scale at the y axis.

1042 KUNSTFELD ET AL THE JOURNAL OF INVESTIGATIVE DERMATOLOGY
allogeneity. The window was defined by time kinetics using a cocktailof chemokines (RANTES, MIP-1, and MCP-1) to ensure optimalT cell inflammation (Fig 1). Injections of the cocktail into skin graftsat day 5 following T cell engraftment did not induce T cell inflamma-tion, after either 6, 24, or 48 h (Fig 1). This cocktail, when injectedat days 7 or 9, was well able to induce inflammation, which was mostpronounced after 48 h (Fig 1). On the other hand, allogeneic T cellresponses (Murray et al, 1994) were absent for up to 7 d followingT cell engraftment and were detectable only in single experiments atday 9. Thus, the following protocol was used for the followingexperiments: chemokines were injected at day 7 following T cellengraftment and T cells were counted 48 h later. All experiments thatshowed any signs of an allogeneic T cell response during the timecourse of the experiment were excluded from further evaluation.Xenoreactivity of human T cells was totally absent within the timeframe of the experiments performed herein.
Under flow MCP-1 or MIP-1α recruit human T cells exclusivelyto human skin To reach sites within human grafts, human T cellshave to use vascular transport. They circulate within the SCID mousevasculature but also travel through the human vessels of the humanskin grafts that have connected to mouse vessels (Murray et al, 1994;Petzelbauer et al, 1996; Kunstfeld et al, 1997). Thus, circulating humanT cells are hit by the chemokine signal under conditions of flow.Under these conditions, the injection of MIP-1α or MCP-1 into
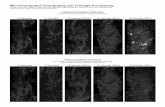
Figure 4. Chemokine-induced patternsof T cell inflammation. (a) Human deepvascular plexus skin 48 h following rh MCP-1 injection; all human vessels in red (UEA-1staining) are surrounded by numerous humanT cells in blue (anti-human CD3 staining,examples given by arrows). The insert in (a)shows human DVP skin 48 h followingsolvent control injection, human vessels inred are surrounded by single human T cellsin blue (arrows). (b) Serial section of the skinspecimen shown in (a); mouse vessels in red(anti-mouse CD31 staining, arrows) are notsurrounded by human T cells in blue (anti-human CD3 staining). (c) Mouse muscle ofthe abdominal wall 48 h following rh IP-10injection; human CD3 positive T cells (red)are attached to and within the peritoneumand between the muscle fibers; the peritonealcavity is marked by an asterisk, counterstaining with Mayer’s hematoxylin in blue.(d) Mouse muscle of the abdominal wall 48 hfollowing solvent control injection, CD3-positive T cells stained in red are completelyabsent; the peritoneal cavity is marked byan asterisk, counter staining with Mayer’shematoxylin in blue. (e) Mouse back skin48 h following rh MCP-1 injection, UEA-1(red) stains the mouse epidermis and mouseadnexial structures but not mouse vessels,CD3-positive human T cells (stained in blue)are completely absent. (f) Mouse glutealmuscle 48 h following rh MCP-1 injection,mouse vessels are stained in red (anti-mouseCD31), CD3-positive human T cells (stainedin blue) are completely absent. Scale bar:0.1 mm.
human skin grafts induced a strong increase in numbers of T cellswithin human skin grafts (mean 58 or 90 T cells per high power field,ranging from 43 to 127 cells), in the order of 10–30 times above thenumbers of cells seen in solvent controls (Figs 2, 3). TNF, which wasused as a positive control, induced similar T cell infiltrates (Fig 2).RANTES, SDF-1, or IP-10 caused only minimal to mild T cellinfiltrates, ranging from 1.3 to 4.5 times (mean) above those of solventcontrol injections (Fig 3, logarithmic scale). The infiltrating T cellswere always in a striking perivascular position to human endothelium.Mouse vessels, even those that had re-vascularized the human skingrafts, were never surrounded by human T cells (Fig 4a, b). None ofthe chemokines was able to recruit T cells into mouse back skin ormouse gluteal muscle (data shown for MCP-1 only; Figs 2, 4). Dosetitration of chemokines for up to 5 µg per injection site did not alterresults, neither in human nor in mouse tissues (data not shown). Wenext tested the effects of mouse MCP-1 on recruitment of humanT cells, which has been shown to bind to and to activate humanT cells even across xenogeneic barriers (Luini et al, 1994). Surprisingly,as seen with human MCP-1, also mouse MCP-1 attracted human Tcells into human skin, again in a perivascular position to humanendothelium, but not into mouse back skin or mouse gluteal muscle(data not shown).
Phenotype of the T cell infiltrates By phenotype, all skin infiltrat-ing T cells were CD45RO1/CD45RA– and CD4 and CD8 T cells

VOL. 111, NO. 6 DECEMBER 1998 CHEMOKINES IN T CELL INFLAMMATION 1043
Figure 5. MCP-1-induced mRNA expression within human and mousetissues. Forty-eight hours following MCP-1 or solvent control injection,mRNA was amplified by RT-PCR from 10 mg of human superficial vascularplexus skin (SVP) and deep vascular plexus skin (DVP), from mouse skin orfrom mouse gluteal muscle.
were equal in numbers in all experiments, irrespective of the chemokineused, indicating that, in our system, there was no preferential chemotaxistowards either T cell subtype (data not shown). By PCR, human IL-2and IFN-γ mRNA were detectable in all chemokine-treated humanskin specimens, but were undetectable or slightly above the detectionlimit in solvent control-treated skin grafts (Fig 5). The presence orabsence of IL-4 mRNA did not follow a distinct pattern, so that wewere unable to conclude that one of the chemokines caused apreferential recruitment of IL-4 producing T cells (data not shown).None of the human cytokine mRNA or the human GAPDH mRNAcould be amplified from mouse back skin or from the mouse glutealmuscle, whether they were chemokine treated or not (Fig 5), con-firming our immunohistochemical findings that human T cells did notmigrate into mouse sites.
Under static conditions, human chemokines attract humanT cells even into mouse tissue To test chemokine function in theabsence of an endothelial barrier or shear stress, chemokines, TNF, orsolvent control were injected into the abdominal wall, directly intothe mouse muscle above the peritoneal cavity, where human T cellshad been injected and were not subjected to flow. At this site, all fivechemokines but not solvent control, attracted numerous human T cellsinto the abdominal wall to an equal extent (absolute numbers of T cellswere in the range of 60–120 cells per high power field). Human T cellswere found at the highest densities within and below the peritoneumand between the adjacent mouse muscle fibers (Fig 4c, d; data shownfor IP-10 only). The T cell density continuously decreased withdistance from the peritoneum.
DISCUSSION
It has been proposed that chemokines largely control for the composi-tion of leukocyte subpopulations in inflammatory conditions. It is thusimportant to rank the proinflammatory ability of chemokines on T cellsin an appropriate assay, which ideally accounts for the complexity ofchemokine interactions with endothelial and T cells, but tries tominimize secondary events due to activation of other white bloodcells. Our Hu-SCID mouse model with grafted human skin and humanT cells appeared optimal, because under conditions described in thispaper, human chemokines did not attract any mouse neutrophils into
human or mouse tissues. Moreover, in this model, the mouse circulationis devoid of human neutrophils, human natural killer cells, or humanmonocytes (Petzelbauer et al, 1996). Thus the observed chemokineeffects on human T cells were not skewed by the activation of otherwhite blood cell subpopulations.
Using this model, we show that among 5-tested chemokines onlyMCP-1 or MIP-1α were able to recruit significant amounts of humanT cells into human SVP and DVP skin sites, whereas RANTES, SDF-1,or IP-10 had only minimal effects on T cell migration. Although severalphenotypic and functional differences have been described between SVPand DVP endothelium (Petzelbauer et al, 1993; Kunstfeld et al, 1997;Lechleitner et al, 1998), such differences do not exist with regard tochemokine-induced T cell recruitment, T cells were equally recruitedto SVP as well as DVP skin in response to the given chemokine. Wecould not discern any specificity of the herein used chemokines towardsa distinct T cell subpopulation, although such differences have beenfound in vitro (Schall et al, 1993; Loetscher et al, 1994; del Pozo et al,1995; Bonecchi et al, 1998; Sieveke and Hamann, 1998). The design ofour experiments, however, clearly does not exclude such differences toexist in vivo, because not necessarily all T cell subpopulations made theirway from the peritoneum into the mouse circulation and because theuse of allogeneic skin and T cells might have obscured subtle differencesof distinct chemokines on T cell subsets.
The other finding that human T cells respond to chemokines onlyat sites of human vessels deserves an additional comment. After singledose injection of chemokines even at concentrations for up to 5 µgper site, none of the used chemokines was able to attract human Tcells across mouse endothelial barriers. This result has to be reconciledwith experiments performed by W. Murphy and D. Taub, whodemonstrated that repetitive s.c. injections of extraordinarily high dosesof MIP-1α, MCP-1, RANTES, or SDF-1 attracted human T cellsinto xenogeneic (mouse) skin (Murphy et al, 1994, 1996; Taub et al,1995b, 1996). We cannot offer a clear explanation, possibly repetitiveinjections caused blood stasis and/or endothelial damage, enabling Tcells to respond to a chemokine gradient similar to that seen in Boydenchamber experiments or to that seen under static (nonflow) conditionsdescribed in our model: the injection of chemokines into the abdominalwall of our SCID mice induced an endothelial cell-independentimmigration of human T cells from the peritoneal cavity through theperitoneum into the abdominal muscle.
The specificity of human T cell recruitment to sites of humanendothelium could be, first, due to the resident cell population withinthe human skin grafts such as mast cells, dendritic cells, etc., whichprovide help for human T cell recruitment at human sites, which isnot provided at mouse tissues. This assumption has to be questioned,because such human resident skin cells were also present around mousevessels that had invaded the human grafts, and T cell infiltrates werenot observed around mouse vessels even at those sites (Fig 4b); second,due to inappropriate interactions of human chemokines with thexenogeneic mouse endothelium resulting in a rapid washout ofchemokines. A detailed analysis of endothelial chemokine receptors ishampered by the fact that they are still not completely characterized.To circumvent this problem, we took advantage of the fact thatchemokine structures are highly conserved (Luo et al, 1997) and bindto and activate T cells even across xenogeneic barriers (Luini et al,1994). We injected mouse MCP-1 into human skin, mouse skin, ormuscle. In spite of the fact that mouse MCP-1 was xenogeneic tohuman endothelium, again human T cell infiltrates were only seen atsites of human endothelium. Because it is unlikely that humanendothelium has a ‘‘better way’’ to interact with human as well asmouse chemokines than mouse endothelium, one has to assume thatchemokines, in order to recruit human T cell under flow, rely onauxiliary endothelial functions, which are given at sites of human butnot of xenogeneic mouse endothelium and that are carried out bymolecules involved in tethering, rolling, or tight adhesion.
In summary, our experiments point towards an important role forMCP-1 and MIP-1α as T cell chemoattractants for skin inflammatoryconditions. Moreover, this MCP-1- and MIP-1α-induced T cellrecruitment to the skin under flow requires auxiliary endothelialfunctions.

1044 KUNSTFELD ET AL THE JOURNAL OF INVESTIGATIVE DERMATOLOGY
This work was supported by grant P10317-MED from the Austrian Science foundation.The authors wish to thank Dr. U.M. Losert and the staff of the Center for BiomedicalSciences of the University of Vienna for animal care and housing, and Dr. B. Pikula ofthe Department of Pathology, University of Vienna for assistance in obtaining human skin.
REFERENCES
Baggiolini M, Dewald B, Moser B: Human chemokines: an update. Annu Rev Immunol15:675–705, 1997
Bazan JF, Bacon KB, Hardiman G, et al: A new class of membrane-bound chemokinewith a CX3C motif. Nature 385:640–644, 1997
Bleul CC, Fuhlbrigge RC, Casasnovas JM, Aiuti A, Springer TA, A: highly efficaciouslymphocyte chemoattractant, stromal cell-derived factor 1 (SDF-1). J Exp Med184:1101–1109, 1996
Bonecchi R, Bianchi G, Bordignon PP, et al: Differential expression of chemokine receptorsand chemotactic responsiveness of type 1 T helper cells (Th1s) and Th2s. J Exp Med187:129–134, 1998
Campbell JJ, Hedrick J, Zlotnik A, Siani MA, Thompson DA, Butcher EC: Chemokinesand the arrest of lymphocytes rolling under flow. Science 279:381–384, 1998
Carr MW, Alon R, Springer TA: The C-C chemokine MCP-1 differentially modulatesthe avidity of beta 1 and beta 2 integrins on T lymphocytes. Immunity 4:179–187, 1996
Kunstfeld R, Lechleitner S, Groger M, Wolff K, Petzelbauer P: HECA-4521 T Cellsmigrate through superficial vascular plexus but not through deep vascular plexusendothelium. J Invest Dermatol 108:343–348, 1997
Lechleitner S, Johnson D, Gille J, Petzelbauer P: Interferon enhances TNF-inducedVCAM-1 (CD106) expression in human endothelial cells by an IRF-1-dependentpathway. J Exp Med 187:2023–2030, 1998
Loetscher P, Seitz M, Clark LI, Baggiolini M, Moser B: Monocyte chemotactic proteinsMCP-1, MCP-2, and MCP-3 are major attractants for human CD41 and CD81T lymphocytes. Faseb J 8:1055–1060, 1994
Luini W, Van Sozzani SDJ, Mantovani A: Species-specificity of monocyte chemotacticprotein-1 and 23. Cytokine 6:28–31, 1994
Luo H, Chaudhuri A, Johnson KR, Neote K, Zbrzezna V, He Y, Pogo AO: Cloning,characterization, and mapping of a murine promiscuous chemokine receptor gene:homolog of the human Duffy gene [letter]. Genome Res 7:932–941, 1997
Murphy WJ, Taub DD, Anver M, Conlon K, Oppenheim JJ, Kelvin DJ, Longo DL:Human RANTES induces the migration of human T lymphocytes into the peripheraltissues of mice with severe combined immune deficiency. Eur J Immunol 24:1823–1827, 1994
Murphy WJ, Tian ZG, Asai O, et al: Chemokines and T lymphocyte activation: II.Facilitation of human T cell trafficking in severe combined immunodeficiency mice.J Immunol 156:2104–2111, 1996
Murray AG, Petzelbauer P, Hughes CCW, Costa J, Askenase P, Pober JS: Human T cell-mediated destruction of allogeneic dermal microvessels in a SCID mouse. Proc NatlAcad Sci USA 91:9146–9150, 1994
Petzelbauer P, Bender JR, Wilson J, Pober JS: Heterogeneity of dermal microvascularendothelial cell antigen expression and cytokine responsiveness in situ and in cellculture. J Immunol 151:5062–5072, 1993
Petzelbauer P, Groger M, Kunstfeld R, Petzelbauer E, Wolff K: Human delayed typehypersensitivity reaction in a SCID mouse engrafted with human T cells andautologous skin. J Invest Dermatol 107:576–581, 1996
del Pozo MA, Sanchez Mateos P, Nieto M, Sanchez Madrid F: Chemokines regulate cellularpolarization and adhesion receptor redistribution during lymphocyte interaction withendothelium and extracellular matrix. Involvement of cAMP signaling pathway.J Cell Biol 131:495–508, 1995
Roth SJ, Carr MW, Rose SS, Springer TA: Characterization of transendothelial chemotaxisof T lymphocytes. J Immunol Methods 188:97–116, 1995a
Roth SJ, Carr MW, Springer TA: C-C chemokines, but not the C-X-C chemokinesinterleukin-8 and interferon-gamma inducible protein-10, stimulate transendothelialchemotaxis of T lymphocytes. Eur J Immunol 25:3482–3488, 1995b
Schall TJ, Bacon K, Camp RD, Kaspari JW, Goeddel DV: Human macrophage inflammatoryprotein alpha (MIP-1 alpha) and MIP-1 beta chemokines attract distinct populationsof lymphocytes. J Exp Med 177:1821–1826, 1993
Sieveke JT, Hamann A: T Helper 1 and T helper 2 cells respond differentially tochemokines. J Immunol 160:550–554, 1998
Taub DD, Lloyd AR, Conlon K, et al: Recombinant human interferon-inducible protein10 is a chemoattractant for human monocytes and T lymphocytes and promotesT cell adhesion to endothelial cells. J Exp Med 177:1809–1814, 1993
Taub DD, Key ML, Clark D, Turcovski Corrales SM: Chemotaxis of T lymphocytes onextracellular matrix proteins. Analysis of the in vitro method to quantitate chemotaxisof human T cells. J Immunol Methods 184:187–198, 1995a
Taub DD, Proost P, Murphy WJ, Anver M, van Longo DLDJ, Oppenheim JJ: Monocytechemotactic protein-1 (MCP-1), 22, and 23 are chemotactic for humanT lymphocytes. J Clin Invest 95:1370–1376, 1995b
Taub DD, Longo DL, Murphy WJ: Human interferon-inducible protein-10 inducesmononuclear cell infiltration in mice and promotes the migration of humanT lymphocytes into the peripheral tissues and human peripheral blood lymphocytes-SCID mice. Blood 87:1423–1431, 1996
Weber C, Alon R, Moser B, Springer TA: Sequential regulation of alpha 4 beta 1 andalpha 5 beta 1 integrin avidity by CC chemokines in monocytes: implications fortransendothelial chemotaxis. J Cell Biol 134:1063–1073, 1996