Low phospholipid-associated cholestasis and cholelithiasis
Transcript of Low phospholipid-associated cholestasis and cholelithiasis

Correspondence.E- mail address: [email protected] (S. Erlinger).
© 2012 Elsevier Masson SAS. All rights reserved.
Clinics and Research in Hepatology and Gastroenterology (2012) 36, S36—S40
COOH
UDCA
H
OH
CH3
CH3
H3C
HO
H H
H
H COOH
CH3
CH3
H3C
OHHO
H
H
H
H
H
NorUDCA
COOHCH3
CH3
H3C
OHHO
H
H
H
H
H
6α-ECDCA
Therapeutic approaches of hepatobiliary Therapeutic approaches of hepatobiliary disorders with ursodeoxycholic aciddisorders with ursodeoxycholic acid
and bile-acid derivativesand bile-acid derivativesGuest editor: L. SerfatyGuest editor: L. Serfaty
C linics and Rd esearch in Hepatology
and Gastroenterology
SEPTEMBER
2012 Supplement 1 - Vol. 36
ISSN
221
0-74
01
7036
4
Disponible en ligne sur
www.sciencedirect.com
SummaryLow phospholipid- associated cholestasis and cholelithiasis (LPAC) is a genetic disorder characterized by cholesterol gallbladder and intrahepatic stones. It is caused by a muta-tion of the gene ABCB4, which encodes the canalicular protein ABCB4/MDR3, a ippase that plays an essential role in the secretion of phosphatidylcholine into bile. Failure of this protein leads to secretion of bile that is poor in phospholipids and, hence, highly li-thogenic, with potent detergent properties. This, in turn, leads to cholangiocyte luminal membrane injury and biliary lesions causing cholestasis. The diagnosis should be suspec-ted when at least two of the following criteria are present: onset of symptoms before the age of 40 years; recurrence of biliary symptoms (biliary colic, jaundice, cholangitis, acute pancreatitis) after cholecystectomy; presence of echogenic foci within the liver indicative of intrahepatic stones or biliary sludge; previous episode(s) of intrahepatic cholestasis of pregnancy; and family history of gallstones in rst- degree relatives. Intra-hepatic stones can be demonstrated by ultrasonography with color Doppler examination, computed tomography and magnetic resonance imaging with magnetic resonance cholan-giography, and the diagnosis con rmed by ABCB4 genotyping. Therapy with ursodeoxy-cholic acid offers prompt relief of symptoms and usually prevents complications. In some cases, however, surgery may be necessary.© 2012 Elsevier Masson SAS. All rights reserved.
Low phospholipid- associated cholestasis and cholelithiasisSerge Erlinger
University of Paris 7- Diderot, 5 rue Thomas- Mann, 75205 Paris cedex 13, France
Abbreviations:
LPAC: Low phospholipid- associated cholestasis and cholelithiasis
UDCA: ursodeoxycholic acid
Introduction
Low phospholipid- associated cholestasis and cholelithiasis (LPAC; OMIM *171060) is a syndrome rst described in 2001 by Rosmorduc, Hermelin and Poupon at the Saint- Antoine Hospital
in Paris [1]. The disorder is caused by a mutation in the class III multidrug resistance/ATP- binding cassette, subfamily B, mem-ber 4 (MDR3/ABCB4) gene, which encodes the bile canalicular protein MDR3. MDR3 (now known as ABCB4) is a member of the superfamily of ABC proteins. It acts as a ippase, moving the phospholipid phosphatidylcholine from the inner lea et of the canalicular membrane to the outer lea et. From there, phosphatidylcholine is washed out into bile by bile acids. Thus, ABCB4 plays a crucial role in the transport of phospha-tidylcholine into bile. Mutations of the gene lead to defective protein that is totally or partially unable to transport this major phospholipid into bile and, therefore, to the secretion of bile
06_Erlinger.indd S36 25/09/2012 11:50:24

LPAC syndrome S37
bile duct stenosis have been reported [5]. This variant may represent 5–10% of cases of LPAC. Otherwise, there are no speci c clinical features, although it shows similar sensitivity to UDCA [5].
When clinical and imaging features are characteristic, there is no doubt as to the diagnosis, and treatment with UDCA may be undertaken without further investigation. In less typical cases or if diagnostic con rmation is needed, a genetic test should be performed. The ABCB4 gene can be analyzed to detect a mutation. It should be noted, however, that only 60–65% of patients have a detectable mutation using the currently available techniques [3]. Thus, the absence of a detectable mutation does not rule out the diagnosis. Point mutations may be heterozygous or homozygous. They can be insertions or deletions and may be nonsense or missense. Missense mutations result in modi cation of the protein with partially preserved function, whereas nonsense (stop codon) mutations result in the production of a truncated protein, with total loss of function. This explains why nonsense mutations are usually associated with more severe disease than are missense mutations. Most mutations are localized to the central part of the molecule near the ATP- binding domain or in adjacent transmembrane domains [3].
without, or with very low levels of, phopholipids. Bile that is poor in (or totally lacking) phospholipids is supersaturated with cholesterol and, thus, highly lithogenic.
Clinical features
In their initial observations, Rosmorduc et al. [1] described six patients (two men and four women, aged 26- 55 years) with a peculiar form of intrahepatic cholelithiasis and a family history of gallstones. All six had a mutation in ABCB4, and all exhibited remarkable improvement on treatment with ursodeoxycholic acid (UDCA). The same authors con rmed these observations in 2003 in 32 patients, mostly women, with a mean age of 38 years [2]. A number of cases have since been published and, although there are no accurate data for its prevalence, it appears that LPAC represents around 5% of patients with symptomatic cholelithiasis.
The diagnosis of LPAC should be suspected when at least two of the following features are present [3]: Onset of symp-toms before the age of 40 years; recurrence of biliary symp-toms (biliary colic, jaundice, cholangitis, acute pancreatitis) after cholecystectomy; presence of echogenic foci within the liver indicative of intrahepatic stones or biliary sludge; previous episode(s) of intrahepatic cholestasis of pregnancy; and a family history of gallstones in rst- degree relatives. However, it should be noted that intrahepatic echogenic foci are only observed in approximately 80- 85% of patients with proven LPAC [3]. This means that the lack of such foci does not rule out the diagnosis.
To these features can be added the remarkable symptoma-tic improvement with UDCA therapy. This observation suggests that symptoms are not directly related to stones, but may also be due to in ammation of intrahepatic bile ducts and to cho-lesterol crystals not detected by ultrasonography. In addition, the bene cial effects of UDCA have been noted clinically well before the stones have been affected. This again suggests that symptoms are not directly related to stones.
Without treatment, the clinical course is severe, with recurrent episodes of cholangitis, jaundice, acute pan-creatitis and, often, biliary cirrhosis. In addition, cases of cholangiocarcinoma have been observed [4, and personal communication with Dr Magali Picon- Coste], and it appears likely that cholangiocarcinoma may complicate the disease, as has been noted in cases of intrahepatic cholelithiasis due to other causes found in, for example, Eastern Asia.
Diagnosis
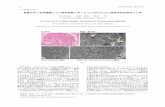
Ultrasound examination of the liver is essential for the diagnosis. This shows intrahepatic stones with acoustic shadows. Color Doppler examination reveals ‘twinkling’ar-tifacts associated with stones (Fig. 1); these artifacts may also correspond to the ‘comet tail’ signs, or images formed by small stones not detected by standard ultrasound (Fig. 2).
Computed tomography (CT) and magnetic resonance (MR) imaging with MR cholangiography (Fig. 3) are also useful for documenting intrahepatic stones. Indeed, these techniques may reveal large bile duct dilatations (Fig. 3). In some cases, large uni- or multifocal spindle- shaped dilatations with no
Figure 1 (A) Standard ultrasound scan shows an intrahe-patic stone with a posterior acoustic shadow (white arrow). (B) Color Doppler ultrasound shows the ‘twinkling’ artifacts (colored bars) associated with stones.
A
B
06_Erlinger.indd S37 25/09/2012 11:50:25

S38 S. Erlinger
easily differentiated from LPAC, a genetic test may prove helpful in such cases.
Pathophysiology
Phosphatidylcholine, the substrate transported by the MDR3 protein, is the major phospholipid of human bile. Phospholipids are responsible, along with bile acids, for cholesterol solubilization and transport in bile. They also protect the biliary epithelium from the detergent effects of physiological hydrophobic bile acids in human bile. Mutations of the ABCB4 gene cause a de cit of MDR3 and a marked decrease in phospholipid concentrations in bile. The rst consequence is the secretion of bile that is supersaturated
To explain the lack of mutations noted in around 40% of patients, it is possible to postulate the presence of muta-tions in an unexplored region of the gene, or in a promoter gene of ABCB4 or another gene encoding, for example, the bile acid transporter or cholesterol transporter.
However, it is noteworthy that mutations of ABCB4 have also been observed in some cases of cholestasis of pregnancy [6], which might explain the possible association between the two diseases. In addition, mutations of ABCB4 are also responsible for progressive familial intrahepatic cholestasis type 3 (PFIC3) [7], a disease of children and young adults frequently associated with cholesterol gallstones.
Differential diagnosis
Two diseases seen in young adults may present with biliary symptoms and intrahepatic stones: primary sclerosing cholangitis; and Caroli’s disease (or congenital dilatation of the intrahepatic bile ducts). Although these are usually
Figure 2 (A) Standard ultrasound scan shows numerous ‘comet- tail’ signs (white arrows) corresponding to small stones or sludge in the left hepatic lobe. (B) Color Doppler ultrasound showing the twinkling arti-facts (colored bars) that correspond to stones not detected by standard ultrasound.
A
B
Figure 3 (A) MRI scan shows a markedly dilated left he-patic duct. (B) MR cholangiography also shows the markedly dilated bile duct. The patient had a severe form of LPAC and bile duct dilatations (see Poupon et al. [5]).
A
B
06_Erlinger.indd S38 25/09/2012 11:50:27

LPAC syndrome S39
This sequence of events has been observed in Mdr2( – /– )
mice. Mdr2 is the murine equivalent of MDR3/ABCB4 in humans. In this mouse model, Mdr2 has been inactivated, and phospholipids are virtually absent from bile. Microcopic examination of gallbladder bile in these animals shows mas-sive amounts of cholesterol crystals. After 12 weeks, 50% of the mice will have developed cholesterol gallstones [8, 9]. Interestingly, female mice have a higher prevalence of cholesterol stones than do males. They also have a more hydrophobic bile salt pool, and it is known that bile salt pool hydrophobicity enhances cholesterol crystallization [8].
with cholesterol and, hence, lithogenic. Cholesterol crystals precipitate in bile and progressively give rise to macros-copic stones. The second consequence is the development of lesions in the biliary epithelium due to the detergent effects of bile acids, which continue to be secreted nor-mally. Without phospholipids, these bile acids form simple micelles with potent detergent properties that can attack cholangiocytic membranes and lead to cellular damage (Fig. 4). In addition, biliary lesions induce cholestasis with high gamma- glutamyltransferase (GGT) concentrations, as GGT is produced mainly by cholangiocytes.
Figure 4 (A) Bile acid, cholesterol and phosphatidylcholine transport through the canalicular membrane in normal hepatocytes. (B) When MDR3/ABCB4 is defective, bile acids are transported without phospholipids. The bile acids then form simple micelles with potent detergent activity that can damage the neighboring cholangiocytes.
A
MDR3/ABCB4
BSEP/ABCB11
BSEP/ABCB11
ABCG5
ABCG5
Mixed micellesPhosphatidyl-cholineBile acidsCholesterol
Bile acidsCholesterol
Simplemicelles
CholangiocytesHepatocyteB
06_Erlinger.indd S39 25/09/2012 11:50:31

S40 S. Erlinger
permits its accurate diagnosis and treatment. Only a few years ago, many of these patients would have been treated with multiple endoscopic interventions and, eventually, by repeat surgery. Knowledge of the LPAC syndrome, its accurate diagnosis by ABCB4 genotyping and its therapy with UDCA offer patients the hope of a disease- recurrence- free future with a simple oral treatment. Genotyping also allows familial screening and treatment of asymptomatic patients before the appearance of complications.
Acknowledgements
The author wishes to thank Professor Didier Mathieu (Service d’imagerie, Fondation Hôpital Ambroise – Paré, Marseille) who kindly provided the imaging documents.
Competing interestsThe author is a consultant for the Mayoly Spindler Laboratories.
References[1] Rosmorduc O, Hermelin B, Poupon R. MDR3 gene defect in
adults with symptomatic intrahepatic and gallbladder cho-lesterol cholelithiasis. Gastroenterology 2001;120:1459- 67.
[2] Rosmorduc O, Hermelin B, Boelle P- Y, Parc R, Taboury J, Poupon R. ABCB4 gene mutation- associated cholelithiasis in adults. Gastroenterology 2003;125:452- 9.
[3] Rosmorduc O, Poupon R. Low phospholipid associated chole-lithiasis: association with mutation in the MDR3/ABCB4 gene. Orphanet J Rare Dis 2007;29:29.
[4] Wendum D, Barbu V, Rosmorduc O, Arrivé L, Fléjou J- F, Poupon R. Aspects of liver pathology in adult patients with MDR3/ABCB4 gene mutations. Virchows Arch 2012;460:291- 8.
[5] Poupon R, Arrive L, Rosmorduc O. The cholangiographic features of severe forms of ABCB4/MDR3 deficiency- associated cholangiopathy in adults. Gastroenterol Clin Biol 2010;34:380- 7.
[6] Bacq Y. Liver diseases unique to pregnancy: a 2010 update. Clin Res Hepatol Gastroenterol 2011;35:182- 93.
[7] Davit- Spraul A, Gonzales E, Baussan C, Jacquemin E. Progressive familial intrahepatic cholestasis. Orphanet J Rare Dis 2009;4:1.
[8] Lammert F, Wang DQ- H, Hillebrandt S, Geier A, Fickert P, Trauner M, et al. Spontaneous cholecysto- and hepatolithiasis in Mdr2- /- mice: a model for low phospholipid- associated cholelithiasis. Hepatology 2004;39:117- 28.
[9] Oude Elferink RP, Ottenhoff R, van Wijland M, Frijters CM, van Nieuwkerk C, Groen AK. Uncoupling of biliary phospholipid and cholesterol secretion in mice with reduced expression of mdr2 P- glycoprotein. J Lipid Res. 1996;37:1065- 75.
[10] Marschall H- U, Wagner M, Zollner G, Fickert P, Diczfalusy U, Gumhold J, et al. Complementary stimulation of hepa-tobiliary transport and detoxi cation systems by rifampicin and ursodeoxycholic acid in humans. Gastroenterology 2005;129:476- 85.
[11] Ikegami T, Matsuzaki Y, Fukushima S, Shoda J, Olivier JL, Bouscarel B, et al. Suppressive effect of ursodeoxycholic acid on type IIA phospholipase A2 expression in HepG2 cells. Hepatology 2005;41:896–905.
[12] Chignard N, Poupon R. Targeting farnesoid x receptor in hepatic and biliary in ammatory diseases. Gastroenterology 2009;137:734–735;discussion 736.
Treatment
LPAC is an excellent indication for UDCA therapy. At an ave-rage dose of 600 mg/d (7–10 mg/kg body weight/d), UDCA is accompanied by a rapid and sustained disappearance of symptoms in the majority of cases [1- 3]. No special diet is helpful, however. In patients with associated hypercholes-terolemia who require medical treatment, statins should be preferred to brates, which increase bile lithogenicity.
As already mentioned above, the effects of UDCA on symptoms can be observed after only a few weeks or months of treatment, when stones have not yet been substantially modi ed by the treatment. This suggests that cholesterol crystals, which disappear rapidly from bile during treat-ment, and/or the associated in ammatory lesions play an important role in the appearance of symptoms.
Precisely when stones do eventually disappear is not known at the present time. In eight patients with large bile duct dilatations treated by UDCA, a decrease in the number of stones was documented after 3 years or more of treatment, and stones were no longer detected by ultrasound and MR imaging in one patient [5]. Several mechanisms may explain the bene cial effects of UDCA: It increases the pool and percentage of hydrophilic bile acids in bile, which protects the membranes of cholangiocytes and, in turn, decreases the toxicity of more hydrophobic bile acids; it increases the expression of MDR3 protein in bile canalicular membranes [10] and, hence, phospholipid secretion in bile; it facilitates cho-lesterol solubilization in bile and, with time, the dissolution of cholesterol crystals and stones; it attenuates proin amma-tory cytokine- induced phospholipase A2- IIa expression, thus inhibiting the in ammatory reaction [11]; and it may prevent phospholipid degradation and attenuate biliary in ammation by enhancing the innate immunity of the biliary tree [12].
So, what are the indications for UDCA treatment in patients with the LPAC syndrome? The list includes those with: symptomatic intrahepatic stones as, in most cases, UDCA allows patients to avoid surgery or endoscopic intervention; asymptomatic intrahepatic stones, as UDCA can prevent the appearance of symptoms or complications. In asymptomatic individuals in whom a mutation of ABCB4 has been detected, but who do not have (or do not as yet have) stones, it would be reasonable to propose periodic screening (for example, a yearly ultrasound examination), and treatment with UDCA only when stones are eventually detected.
As a treatment, cholecystectomy may be necessary in cases of gallbladder stones complicated by recurrent biliary colic or acute cholecystitis. However, if there are no macroscopic stones in the gallbladder, but only symptomatic biliary sludge, then cholecystectomy may be deferred, and UDCA treatment attempted instead.In a few cases of intrahepatic stones and marked dilatations of bile ducts, biliary drainage or even partial hepatectomy may be necessary [5]. In more advanced cases with decompensated biliary cirrhosis with permanent jaundice or ascites, liver transplantation should be considered.
ConclusionLPAC is a remarkable example of the bene ts that molecular biology can bring to clinical practice. The technology has allowed the identi cation of a new syndrome, and now also
06_Erlinger.indd S40 25/09/2012 11:50:31