Third Edition Chapter 2: Describing Location in a Distribution
Location, location, location: identifying the neighborhoods of LPS signaling
Transcript of Location, location, location: identifying the neighborhoods of LPS signaling

Toll-like receptors (TLRs) are transmem-brane proteins that, after microbial
ligand–induced formation of oligomers, recruit specific signaling adaptors to their cytoplasmic Toll–interleukin 1 (IL-1) recep-tor domains1. These adaptors initiate a chain of phosphorylation and ubiquitination reac-tions that eventually lead to activation of the transcription factor NF-κB, mitogen-acti-vated protein kinases (MAPKs) and inter-feron-response factors (IRFs). Unique among the large family of TLRs, TLR4 engages two distinct adaptor proteins: MyD88, which is recruited by TIRAP (also called Mal) and elicits the production of proinflammatory cytokines; and TRIF, which is recruited by the adaptor TRAM and activates the produc-tion of type I interferons as well as proinflam-matory cytokines. As TIRAP and TRAM are thought to bind to a common motif on TLR4 in dimers2, precisely how TLR4 coordinates recruitment and signaling through two poten-tially competing adaptor systems remains unclear. In this issue of Nature Immunology, Kagan et al. propose a solution to this puzzle by showing that TLR4 signals through TIRAP-MyD88 and TRAM-TRIF sequentially rather than simultaneously3. Their data indicate that endocytosis of TLR4 terminates an ini-tial phase of MyD88-dependent signaling and heralds the start of a second phase of TRIF-dependent signal transduction from TLR4 molecules located in endosomes. In this sce-nario, TLR4 ‘steps into line’ with TLR3, TLR7,
Location, location, location: identifying the neighborhoods of LPS signalingColin Watts
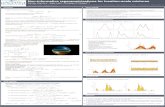
TLR4 somehow manages to activate both MyD88- and TRIF-dependent signaling. According to Medzhitov and colleagues, TLR4 engages MyD88 and TRIF sequentially at the cell surface and in endosomes, respectively, thereby providing access to distinct pools of signaling proteins.
TLR8 and TLR9, which activate IRF3 and IRF7 for production of type I interferons and which all signal from an intracellular location. This ‘geographical’ constraint may be imposed by the intracellular location of TRAF3, an adap-tor required for the transmission of MyD88 and TRIF signals that induce interferon production4.
Kagan et al. use several new tools to manip-ulate the TLR4 signaling pathway. First, they interfere with the normal lipopolysaccharide (LPS)–induced endocytosis of TLR4 (ref. 5) by incubating macrophages with ‘dynasore’, an inhibitor of the GTPase dynamin, which is required for the completion of clath-rin-mediated endocytosis and some other forms of endocytosis6. Second, they extend the work of other labs7 on the membrane localization of the TRIF-associated adap-tor TRAM by generating a mutant form of TRAM that is targeted to endosomes but not to the plasma membrane. They show that in bone marrow–derived macrophages exposed to LPS, the presence of dynasore ablates sev-eral TRIF-TRAM–dependent signals, includ-ing those inducing phosphorylation of IRF3 and the production of IL-6 and interferon-β. However, they find that MyD88-dependent activation of the MAPK p38 and NF-κB-dependent resynthesis of the NF-κB inhibi-tor IκBα are maintained, albeit to a somewhat lesser degree. These results indicate that a dynamin-dependent process, presumably the endocytosis of TLR4, is necessary for com-plete LPS signaling and that TLR4 trapped on the cell surface can engage and activate the TIRAP-MyD88 pathway but not the TRAM-TRIF pathway (Fig. 1).
Studies have shown that amino-terminal myristoylation of TRAM is essential for the
reconstitution of LPS-induced production of IL-6 and the chemokine RANTES in TRAM-deficient macrophages; restored LPS respon-siveness correlates with targeting of TRAM to the plasma membrane and localization together with TLR4 (ref. 7). Testing earlier predictions that TRAM, like other amino-myristoylated proteins, requires additional signals for membrane targeting7, Kagan et al. identify three basic residues necessary for tar-geting of TRAM to the plasma membrane and binding of phosphoinositides. TRAM lacking this polybasic motif but retaining its myris-toyl group still localizes to endosomal mem-branes, a subcellular location for TRAM not obvious in earlier studies. Production of IL-6 and RANTES is successfully reconstituted in TRAM-deficient macrophages expressing this version of TRAM, consistent with the propo-sition that the TRIF-dependent, MyD88-inde-pendent arm of TLR4 signaling takes place from an endosomal rather than a plasma membrane platform.
Thus, like TLR3, TLR7, TLR8 and TLR9, TRAM drives the production of type I inter-feron from an intracellular location. Why must these TLRs signal from endosomes? Adaptor proteins do not seem to constrain TLR signaling to specific subcellular loca-tions, as TLR7 and TLR9 are MyD88 coupled, whereas TLR3 signals through TRIF. Kagan et al. propose that plasma membrane–local-ized TLRs such as TLR2 do not induce the production of type I interferon, because TRAF3, an essential adaptor of both MyD88 and TRIF pathways leading to interferon production4, is located in the cytosol and does not readily access plasma membrane signaling complexes. Confirming that idea, they show that a mutant TRAF3 linked to the
NATURE IMMUNOLOGY VOLUME 9 NUMBER 4 APRIL 2008 343
Colin Watts is in the Division of Cell Biology and
Immunology, Wellcome Trust Biocentre, College of
Life Sciences, University of Dundee, Dundee DD1
5EH, UK.
e-mail: [email protected]
N E W S A N D V I E W S©
2008
Nat
ure
Pub
lishi
ng G
roup
ht
tp://
ww
w.n
atur
e.co
m/n
atur
eim
mun
olog
y

phosphatidylinositol-3,4-bisphosphate (PtdIns(4,5)P2)–binding domain of phospho-lipase C-δ is partially redirected to the plasma membrane and permits the unusual induction of interferon-β by a TLR2 ligand.
Kagan et al. propose an elegant solution to the problem of how TLR4 couples to two different Toll–IL-1 receptor domain–contain-ing adaptors. New questions can now be for-mulated that will further test and refine the model. First, the data are consistent with, but do not formally prove, sequential rather than simultaneous recruitment of the two adap-tor complexes. TRAM-TRIF complexes might be recruited to cell surface TLR4 but might be unable to signal until access to TRAF3 is achieved in endosomes. That possibility might be assessed by analysis of whether the aforementioned plasma membrane–targeted TRAF3 ‘rescues’ the TRAM-TRIF signaling arm of cell surface–trapped TLR4. A ‘sequen-tial adaptor’ model predicts that it will not. Additional support for the idea of sequential
adaptor recruitment would be obtained if it is found that immunoprecipitated ‘dynasore-stalled’ cell surface TLR4 engages TIRAP-MyD88 but not TRAM-TRIF complexes and if immunoprecipitation over a time course of LPS-triggered TLR4 endocytosis shows sequential association first with TIRAP-MyD88 and only later with TRAM-TRIF. Such a kinetic analysis could be combined with selective isolation of TLR4 signaling complexes from the cell surface and from intracellular compartments, as has been done to investigate compartment-specific signaling in the epidermal growth factor receptor sys-tem8.
In addition, these findings await integration with data identifying other TLR4 signaling complexes that form sequentially. The tumor necrosis factor family member 4-1BBL is required for sustained production of tumor necrosis factor in macrophages9. 4-1BBL synthesis is induced by LPS and 4-1BBL is pre-cipitated together with TLR4 around the time
that MyD88 is lost from the TLR4 complex. Although it is possible that TLR4 associates sequentially with MyD88, then TRIF and then 4-1BBL to drive distinct waves of signaling, the cell surface location of 4-1BBL indicates either that endocytosed TLR4 recycles at later times to engage 4-1BBL or, perhaps more likely, that a new population of TLR4 is involved in this ‘downstream’ signaling complex.
Dynasore is fully reversible, which means that inhibitor ‘washout’ experiments should now allow a delay time to be inserted between the MyD88- and TRIF-dependent phases of LPS-driven signaling. The duration and tim-ing of TLR signaling is crucial for proper regulation of dendritic cell responses10, so it will be interesting to see which responses still occur and which do not when the ini-tial MyD88 and subsequent TRIF signals are separated not only compartmentally but also temporally.
How might the MyD88 arm of LPS signal-ing be terminated after TLR4 endocytosis? Kagan et al. propose that the turnover of PtdIns(4,5)P2 after scission of vesicles from the cell surface releases TIRAP, shown before to be recruited by this phosphoinositide. An alternative or additional possibility may be that the signaling partners of TLR4 are neces-sarily ‘reset’ as a requirement of the endocytic process. Receptor endocytosis, at least through clathrin-coated pits, requires the engagement of an endocytic adaptor such as AP2 or one of the other clathrin-associated sorting pro-teins11. The binding of endocytic adaptors to TLR4 might be mutually exclusive with the binding of Toll–IL-1 receptor domain adap-tors, making dissociation of TIRAP-MyD88 complexes a precondition of entry into coated pits. Moreover, endocytic adaptors also bind to PtdIns(4,5)P2, as does dynamin itself, thus offering additional potential ways to displace TIRAP at the site of TLR4 endocytosis.
The idea that TLR4 enters cells by clath-rin-mediated endocytosis is supported by an earlier study showing that ‘knockdown’ of clathrin suppresses LPS uptake in fibro-blasts5. However, other modes of endocytosis are also dynamin dependent, so it is possible that TLR4 uptake proceeds by more than one pathway and that TLR4 may become local-ized to more than one type of endocytic structure. TLR4 ligation is known to rapidly stimulate actin-dependent macropinocytosis and phagocytosis in dendritic cells12, which might further enhance TLR4 endosomal sig-naling. Indeed, some of the TRAM-express-ing, dextran-filled endosomes in the study of Kagan et al. resemble macropinosomes rather than conventional endosomes. Further delin-eation of the processes required for TLR4
344 VOLUME 9 NUMBER 4 APRIL 2008 NATURE IMMUNOLOGY
++
++++
++
Plasmamembrane
TRAM
TIRAP
TLR4
LPS
LPS
LPS
LPS
?
PIP2
IRAK4,IRAK1
TIRAP-MyD88
MyD88
TRIF
Dynamin
Endosome
CD14
TRAF6
TRAF3
TAK1
NF-κB, MAPKs
NF-κB, MAPKs
RIP1-TRAF6
IκBαIL-6, IL-12
TBK1 + IKKi
TRAM-TRIFIRF3
IFN-β
Early
Late
Figure 1 Sequential engagement of MyD88 and TRIF adaptors by TLR4. Kagan et al. propose that TLR4 signals through the TIRAP-MyD88 adaptor pair from a cell surface location to activate both p38 and limited NF-κB-dependent transcription. LPS triggers endocytosis of TLR4 through one or more dynamin-dependent pathways, leading to the termination of MyD88 signaling, possibly because of displacement of TIRAP-MyD88 by endocytic adaptors (not presented here for clarity). In endosomes, a second phase of TRAM-TRIF–mediated signaling is initiated, aided by improved access to TRAF3. This leads to production of interferon-β (IFN-β) after IRF3 activation and to a second phase of MAPK and NF-κB signaling required for the production of inflammatory cytokines. PIP2, PtdIns(4,5)P2; IRAK, TAK1, RIP1, TBK1 and IKKi, kinases.
Kim
Cae
sar
NEWS AND V IEWS©
2008
Nat
ure
Pub
lishi
ng G
roup
ht
tp://
ww
w.n
atur
e.co
m/n
atur
eim
mun
olog
y

internalization and the identification of the types of endosomes to which TLR4 has access is probably worthwhile. Conceivably, CD14 may be involved in TLR4 endocytosis, as at least superficially, the LPS-signaling capa-bilities of ‘dynasore-arrested’ macrophages resemble those of CD14-deficient macro-phages that, when challenged with ‘rough’ LPS, activate MAPKs and NF-κB but fail to produce type I interferon13.
The idea that TRIF-dependent signaling from TLR4 occurs subsequent to MyD88-dependent signaling from the same receptor is consistent with earlier data showing that the kinetics of p38 phosphorylation and NF-κB
activation are delayed in cells lacking MyD88 and are more sustained when both pathways are intact14,15. Indeed, by organizing the two TLR4 adaptor systems to deliver sequential signals from different signaling platforms, dendritic cells may be better able to integrate and sus-tain key signaling events such as activation of NF-κB and MAPK. As discussed elsewhere in the context of synergy among various TLR stimuli, by making endocytosis of microbial material the permissive event for a second amplifying phase of signaling, dendritic cells can link the eventual presentation of microbial antigens to the optimal expression of T cell–polarizing molecules such as cytokines10.
1. O’Neill, L.A. Curr. Opin. Immunol. 18, 3–9 (2006). 2. Nunez Miguel, R. et al. PLoS ONE 2, e788 (2007). 3. Kagan, J.C. et al. Nat. Immunol. 9, 361–368
(2008). 4. Hacker, H. et al. Nature 439, 204–207 (2006). 5. Husebye, H. et al. EMBO J. 25, 683–692 (2006). 6. Macia, E. et al. Dev. Cell 10, 839–850 (2006). 7. Rowe, D.C. et al. Proc. Natl. Acad. Sci. USA 103,
6299–6304 (2006).8. Burke, P., Schooler, K. & Wiley, H.S. Mol. Biol. Cell
12, 1897–1910 (2001).9. Kang, Y.J. et al. Nat. Immunol. 8, 601–609 (2007). 10. Macagno, A., Napolitani, G., Lanzavecchia, A. &
Sallusto, F. Trends Immunol. 28, 227–233 (2007). 11. Maldonado-Baez, L. & Wendland, B. Trends Cell Biol.
16, 505–513 (2006).12. West, M.A. et al. Science 305, 1153–1157 (2004).13. Jiang, Z. et al. Nat. Immunol. 6, 565–570 (2005). 14. Yamamoto, M. et al. Science 301, 640–643 (2003). 15. Hoebe, K. et al. Nature 424, 743–748 (2003).
NATURE IMMUNOLOGY VOLUME 9 NUMBER 4 APRIL 2008 345
New niches for B cellsTakashi Nagasawa
The bone marrow contains specialized microenvironments that maintain blood cells and supply the requisite factors for their development. Newly identified bone marrow–resident dendritic cells create unique niches for mature B cells.
Takashi Nagasawa is in the Department of
Immunobiology and Hematology, Institute for
Frontier Medical Sciences, Kyoto University,
Shogoin, Sakyo-ku, Kyoto 606-8507, Japan.
e-mail: [email protected]
Bone marrow occupies the medullary cavi-ties of bones throughout the skeleton.
Diverse lineages of blood cells, including B cells and their precursors, arise from com-mon hematopoietic stem cells and develop in a complex microenvironment in the densely cellular spaces in the bone marrow. Thus, elu-cidating the spatial microenvironmental orga-nization and the interaction between blood cells and the microenvironment is important for understanding the regulatory mechanisms of lymphohematopoiesis. It has been assumed that the bone marrow contains cells that cre-ate the special microenvironments known as ‘niches’ that maintain blood cells and supply the requisite factors for their development and maintenance. Although the lack of distinctive characteristics of nonhematopoietic cells in bone marrow makes it difficult to identify the cells that create niches for hematopoiesis (cellular niches), studies suggest that non-hematopoietic cells, including a population of osteoblasts lining the bone surface (called ‘SNO cells’1), endothelial cells2, and/or a pop-ulation of reticular cells with long processes (called ‘CAR cells’3) constitute cellular niches for hematopoietic stem cells. For B cells, CAR
cells are suggested to function as niches for very early B cell precursors and end-stage B cells (plasma cells)4. In B cell development, bone marrow is known to act as the primary lymphoid organ, but studies have shown that mature B cells in the bone marrow reside near the vasculature and participate in T cell– independent immune responses to blood-borne microbes, including salmonella5. However, the cellular components of the peri-vascular niches have remained elusive. A study in this issue of Nature Immunology shows that perivascular clusters of dendritic cells (DCs) function as niches for mature B cells in the bone marrow6. Although macrophages are thought to be important components of the bone marrow microenvironment, their func-tions in hematopoiesis in vivo have remained elusive. Thus, this is the first demonstration that hematopoietic cells function as the niche for blood cells in the marrow.
The bone marrow contains a dense net-work of small blood vessels called ‘vascular sinuses’ (Fig. 1a). The nutrient artery pen-etrates the cortex of bone, and descending arteries become cortical capillaries that enter the vascular sinuses. The sinuses eventually enter the central sinus, which enters the sys-temic venous circulation. Blood cells and their precursors are packed in the extravas-cular spaces between the vascular sinuses, and among these cells, hematopoietic stem cells, mature B cells and megakaryocytes (from which platelets are generated) are
associated with the endothelium in the bone marrow2,5,7.
The DCs also known as ‘conventional DCs’ are the most powerful antigen-presenting cells, which extend large processes or ‘veils’ and are key in triggering T cell–dependent immune responses. Most tissues, including spleen and lymph nodes, are populated with DCs. Sapoznikov and colleagues have now iden-tified bone marrow-resident DCs (bmDCs) that have the ability to prime naive T cells like splenic DCs do but differ from splenic DCs by high expression of αE integrin (CD103) and the monocyte marker F4/80 (ref. 6). They visualize bmDCs in the cranial bone marrow using mice in which the gene encod-ing the chemokine CX3CR1 is replaced with a green fluorescent protein (GFP) reporter gene (called ‘CX3CR1-GFP’ mice); notably, in situ imaging analysis shows that bmDCs are orga-nized in perivascular clusters surrounding the distinct vascular sinuses6. In addition, after transferring labeled mature B cells into the spleens of CX3CR1-GFP mice, they find that the grafted B cells home to the perivascular area covered by bmDCs6.
To clarify the functions of bmDCs in devel-opment of B cells, Sapoznikov and colleagues analyze mice that can ablate DCs with high expression of CD11c after being given diph-theria toxin6. The bmDC-depleted mice have many fewer mature B cells in the bone mar-row, although the numbers of B cell precur-sors in the bone marrow and mature B cells in
NEWS AND V IEWS©
2008
Nat
ure
Pub
lishi
ng G
roup
ht
tp://
ww
w.n
atur
e.co
m/n
atur
eim
mun
olog
y