Invivo β-adrenergic induction of the unmasking of the uncoupling protein in rat brown fat
Transcript of Invivo β-adrenergic induction of the unmasking of the uncoupling protein in rat brown fat

Camp. Biockm. Physiol. Vol. 106C. No. I, pp. 171-177, 1993 Printed in Great Britain
0742-8413/93 $6.00 + 0.00 Q 1993 Pergamon Press Ltd
IN VIVO p-ADRENERGIC INDUCTION OF THE UNMASKING OF THE UNCOUPLING PROTEIN
IN RAT BROWN FAT
MARC GOUBERN,* MARIE-FRANCE CHAPEY, * MARIE-CLAUDE LAuRYt and RENB PORTET*
‘Laboratoire d’Adaptation Energktique $ l’Environnement, EPHE, 11 place Marcelin Berthelot, 75231 Paris cedex 05, France; and TLaboratoire de Physiopathologie de la Nutrition, CNRS URA 307,
Universiti Paris VII, Paris, France.
(Received 11 March 1993; acceptedfor publication 14 April 1993)
Abstract-l. In 28°C adapted rats (WA) both cold stress and norepinephrine (NE) led to a 4-fold increase of uncoupling protein dependent proton conductance which was abolished by propranolol (PRO).
2. In 4-day warm re-exposed rats (after 10 days at 5°C) (WR) the same uncoupling by cold stress was observed but the NE effect was lower. Uncoupling by cold stress was not abolished by PRO.
3. In WR rats, uncoupling was not due to the involvement of an a-adrenergic pathway. 4. Both P-agonist isoproterenol and /I3-agonists BRL 35135A and ICI D71 I4 led to high levels of
unmasking. 5. Interscapular brown adipose tissue surgical denervation, which abolished cold stress unmasking both
in WA and WR rats, indicates a mediation by direct sympathetic innervation. 6. Depending on the thermal history of the rat, the possibility that unmasking by cold stress could be
mediated by different types of b-receptors is discussed.
INTRODUCTION
Brown adipose tissue (BAT) is the major site of both non-shivering thermogenesis and diet-induced ther- mogenesis (Smith 1961, Himms-Hagen, 1985). It has been found to be a common effector for both thermic and weight regulation (Ricquier and Mory, 1984). In this tissue, the respiration rate is controlled by an uncoupling protein (UCP) located in the inner mito- chondrial membrane which acts as a proton channel (Shrago and Strieleman, 1987; Klaus et al., 1991). The proton extrusion linked to substrate oxidation is dissipated to produce heat. Parallel measurements of GDP binding and UCP content have clearly shown that acute stimulation of thermogenesis in the rat by cold stress or by norepinephrine (NE) injection leads in neither case to an increase in UCP content but does induce a rapid unmasking of the nucleotide binding sites which regulate its activity (review in Trayhurn and Milner, 1989). The mechanisms involved in this well known phenomenon have not been established.
Some attempts to correlate the increased GDP binding induced by unmasking with GDP sensitive mitochondrial swelling, which is a semiquantitative functional technique, have shown varied responses (Swick and Swick, 1986, Trayhurn ef al., 1987, Peachey et al., 1988). However, a recent direct func- tional quantification using a comprehensive chemios- motic approach has clearly demonstrated a
Address correspondence to: M. Goubern, Laboratoire de Nutrition et Stcuritt Alimentaire, INRA, 78352 Jouy- en-Josas Cedex, France. (Fax 16-I-3465-231 1)
masking/unmasking capacity of effective proton con- ductance over one order of magnitude (Goubern et al., 1992).
The development of thermogenesis in BAT is under the control of hypothalamic thermoregulatory cen- ters, and the effector pathway involves the sympath- etic system via the release of norepinephrine (NE) (Cottle and Cottle, 1970, Derry et al., 1969). Several pharmacological studies on animals or cultured cells have demonstrated that UCP synthesis is under nor- adrenergic control with some synergism between B and a receptors (Jacobson er al., 1986, Rehnmark et al., 1990). Concerning acute UCP unmasking, two studies have shown some increase in GDP binding after acute administration of two recent /l adrenore- ceptor agonists (Milner et al., 1988, Granneman and Lahners, 1992) but no comparative study with classi- cal reference agonists has been made. Such a study is significant because there is evidence for the presence of no less than four types of adrenergic receptors in BAT (a 1, ct2, p 1 and fi3), each of which could be implicated in the adrenergic control of UCP unmask- ing (Girardier and Seydoux, 1986; Arch, 1989). Con- sequently, pharmacological characterization of the response in uiuo has been attempted here. The present experiments share the inherent limitation of all in vim
studies in that secondary effects may be involved but, to the best of our knowledge, it is not possible to mimic adrenergic control of UCP unmasking in vitro.
The same UCP masking profile has been observed both in warm adapted rats at thermal neutrality and in cold exposed rats when returned to a warm envi- ronment for some hours or some days and this is in
171

172 M. GOUBERN et at.
spite of a great difference in UCP content (Goubern et al., 1992). Thus, the thermal history of the rat has been examined to establish its effect on the unmask- ing response to adrenergic stimulation.
MATERIALS AND METHODS
Animals and diets
Male Long-Evans rats reared at 23°C received ad ~ibitu~ a standard laboratory diet (UAR A03): pro- teins 23.5%, lipids 5%, carbohydrates 49.8%.
Warm adapted and warm re-exposed rats were used to modulate the thermal past of the animals. Warm adapted rats [a group of rats (7 weeks of age)] were exposed at 28°C (thermal neutrality) for at least 4 weeks. Warm re-exposed rats [a group of rats (9 weeks of age)] were exposed to cold (5OC) for 10 days and then warm re-exposed at 28°C for 4 days.
BAT was denervated to ascertain the role of BAT sympathetic innervation in the unmasking process. After 4 weeks of exposure at 28°C interscapular BAT was surgically denervated (animals were anesthetized with chloral hydrate (300 mg/kg ip.), the five pairs of nerves supplying the two BAT lobes were isolated and cut without damage to the tissue). Rats were killed 4 days after denervation. In the warm re- exposed group, surgical sympathectomy was per- formed after 10 days of cold exposure, and then they were re-exposed at 28°C for 4 days.
For unmasking of the proton channel of the uncou- pling protein, warm adapted rats or warm re-exposed rats were acutely injected, 1 hr before killing with various CI- or /?-adrenergic agonists or antagonists. When assayed, antagonists were administered 20 min before the t hr unmasking, resulting from cold stress at 5°C or from injection of agonists. Doses were selected which gave maximal response; they are indicated in the results section.
Interscapular BAT was rapidly dissected out and used for experiments.
Isolation of ~itoc~ondr~a
Mitochondria were isolated by Cannon and Lind- berg’s method (1979). After the final centrifugation, they were kept in 250mM sucrose, 5 mM TES, pH 7.2.
Respiratory studies
Oxygen consumption and membrane potential were determined simultaneously. Oxygen uptake was measured polarographically with a Clark-type elec- trode and membrane potential with a laboratory constructed tetraphenylphosphonium (TPP+ ) selec- tive electrode (Kamo et al., 1979). measurements were carried out in a medium containing 1OOmM sucrose, 20 mM TES, 4 mM K,HPO,, 2 mM MgCIZ, 1 mM EGTA, 1% w/w fatty acid free serum albumin and 5 ,rrM rotenone (final volume 1.5 ml). 0.5 mg mitochondrial protein was added per assay. Respir-
ation rate was modulated by varying the a-glyc- erophosphate substrate concentration. Being dependant on the native proton conductance, state 4, which is investigated here, provides a valuable cri- terion for the assessment of BAT thermogenic activity (Nicholls, 1982).
Membrane potential was corrected to take into account the activity coefficient of TPP+ in the matrix according to Rottenberg (1984) (about 50 mV in these experimental conditions).
Calorigenic effect of isoprenaline (ISO)
The experiments were carried out on unanes- thetized and unrestrained rats which had fasted for 6 hr. Measurement was performed at 25°C. A con- tinuous record of oxygen consumption was kept using an oxygen analyser (Beckman, Munchen, FRG) following IS0 injection (200 pg/kg i.m.).
Statistics
Data were expressed as mean + SEM. The signifi- cance of the differences between different groups was analyzed using Student’s t-test.
Materials
(-)Arterenol bitartrate (norepinephrine), DL
propranolol hydrochloride, (-)isoproterenol hydro- chloride (isoprenaline), L-phenylephrine hydrochlor- ide, yohimbine hydrochlo~de were all obtained from Sigma. BRL 35135A (Beecham Lab., U.K.), ICI D7114 (ICI Pharm., U.K.), prazozin hydrochloride (Pfizer Lab., France) and pentholamine hydrochlor- ide (Ciba Geigy, France) were generous gifts.
RESULTS
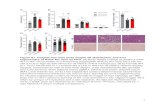
Cold stress or acute injection of NE are the two classical conditions known to unmask UCP. Figure 1A draws a comparison of typical exper- iments. Values of membrane potential are plotted against values of respiration rate which was modu- lated by varying substrate concentration. In warm adapted rats or in 4 days warm re-exposed rats (after 10 days at SC), respiratory rates and membrane potential were found to be similar. Comparison of force-flux relationships clearly showed the same large decrease in membrane potential over the whole range of crGP oxidation after UCP unmasking by cold stress (1 hr at 5°C). Acute injection of NE (200 pg/kg i.m., 1 hr before killing) led to the same level of uncoupling of mitochondrial respiration in warm adapted rats while warm re-exposed rats exhibit a much lower UCP unmasking. This lower unmasking is not enhanced by a higher dose of NE (400pg/kg i.m., acutely injected to warm re-exposed rats. These changes in membrane potential (depolarization) in- duced by the unmasking process are parallelly shifted independently of the respiration rate (Fig. 1B).
From these measurements, it is possible to calcu- late UCP dependant proton conductance (CmH+ )

Uncoupling protein in brown fat mitochondria 173
according to the method largely described in previous publications (Goubem et al., 1990, 1991). Although it has previously been estimated that there is 2.3 times more UCP in the mitochondria of warm re-exposed rats than those of warm adapted rats (Goubern et al., 1992), cold stress led to the same 4-fold increase in CmH+. Acute NE injection led to the same CmH+ increase in warm adapted rats, whereas in warm re-exposed rats, only a 2-fold CmH+ increase is observed (results not shown).
Figure 2 shows comparison of time-course vari- ations in BAT UCP unmasking and the calorigenic effect in uivo of the potent P-agonist isoproterenol (ISO) in warm re-exposed rats. Unmasking (Fig. 2A) was as rapid and as reversible as the increase in O2 consumption (Fig. 2B). Significant BAT mitochon- drial depolarization was observed within 10 min and disappeared after 2-3 hr, the same as for the increase in whole body 0, consumption. This experiment indicates clearly that the high unmasking level ob- tained by injection of an acute dose of the /?-agonist is very rapid, and thus does not require significant UCP synthesis or insertion in BAT mitochondria, which confirms previous measurement of UCP con- tent (Goubern et al., 1992). Comparable time-course
Membrane potential (mV)
P
Respiratory rate
(nmolOZ.min-‘.mg-Iprot.)
50
0 50 100
Depolartzatton (mV)
-50
I ?=!+i*_i 6 .-*_ - - b
I y-y-9-p-y-q-e-7
SO
Fig. 1. (A) Representative variations of membrane potential with respiration rate in brown adiuose tissue mitochondria. Respiration rate was modulated with a-glycerophosphate additions (final concentration 0.1-10 mM). (B) Variations of membrane depolarization with respiration rate. Six animals per group; values are mean f SEM. Black symbols are for warm adapted rats and white symbols are for 4-day warm re-exposed rats (after 10 days at 5°C). Controls (A, A). Unmasking by cold stress (1 hr at 5°C) (0, 0) or by
norepinephrine injection (1 hr at 28°C) (m, 0).
OJ
0 60 120 180
I Tima Imint
IS0
Fig. 2. Comparison of representative time-course variations of membrane potential with respiration rate in brown adipose tissue mitochondria after UCP unmasking (A) and of calorigenic effect in vivo (% increase in 0, consumption) (B) by isoproterenol injection to warm re-exposed rats
(200 pg/kg i.m.).
variations could be observed with acute injection of norepinephrine (NE).
Comparison of the effects of the different treat- ments can be easily performed by membrane depolar- ization which indicated mitochondrial uncoupling. As the lines of membrane depolarization are practi- cally parallel, only values corresponding to a respirat- ory rate of 50 nmol0, were reported to simplify comparison.
IBAT of warm adapted or warm re-exposed rats was surgically denervated to ascertain if BAT sym- pathetic innervation is directly implicated in the unmasking process. Sympathectomy does not modify membrane potential in IBAT of warm adapted or warm re-exposed control rats. Figure 3 shows that depolarization by cold stress was reduced by about 75-lOO%, which indicates that unmasking is mainly mediated by direct sympathetic BAT innervation. As shown by the response to NE or IS0 injection which was not modified compared to control rats, unmask- ing capacity is maintained after IBAT surgical denervation.
In warm adapted rats (Fig. 4), unmasking by cold stress or acute injection of NE could be mimicked by

M. GOUBERN er al. 174
Depolarization (mV)
.50
0
W.ii
ffl SY S‘Y SY 9 SY 9 SY SY
5” NE IS0 5” NE IS0
Fig. 3. Variations of membrane depolarization in mitochon- dria of denervated interscapular brown adipose tissue of warm adapted rats (W.A.) and of 4-day warm m-exposed rata (after 10 days at 5°C) (W.R.). lnt~~puiar brown adipose tissue was surgically denervated 4 days before UCP unmasking either by cold stress (I hr at ST) or by iso- proterenol (ISO) or norepinephrine (NE) injection. Six
animals per groups; values are mean + SEM.
the acute injection of the selective /l-agonist IS0 (2~~g kg i.m., 1 hr before sacrifice). The potent p-antagonist propranolol (PRO) administered 20 min before unmasking (10 mg/kg i.p.) completely abol- ished the NE effect and considerably reduced depolarization resulting from cold stress.
In warm re-exposed rats, IS0 injection led to a membmne de~iari~tion double that observed for cold stress. Such depolarization by IS0 corresponds
to a CmH + 7 times that observed in controls (results not shown). NE associated with cold stress was synergistic and led to increased depolarization. Only NE unmasking was compIetely abolished by propra- nolol; IS0 depolarization was halved and, surpris- ingly, propranolol had absolutely no effect on the cold stress group. Repeated administration of pro- pranolol (IO mg per day during 2 days) before cold stress led to the same lack of effect. Thus, comparison revealed greater unmasking potential in the warm re-exposed group compared to warm adapted rats as well as varying effects of propranolol. IS0 is the most potent unmasking agent used here.
The lack of effect of propranolol on unmasking by cold stress in warm m-exposed rats led investigation as to whether an a-adrenergic pathway could be involved in this process (Fig. 4). Selective xl stimu- lation by phenylephrine (PHE) (5mgikg i.p., 1 hr hefore sacrifice) had no effect. Results showed that it is unlikely that z I or r2-adrenergic receptors partici- pate in unmasking by cold stress or NE injection, since injection of different 3 antagonists (phento- lamine CL (PE), prazosin u 1 (PZ) or yoh~mbin a2 (YO)] 5 mgjkg i.p. 20 min before the 1 hr unmasking had absolutely no effect. Thus, the r-adrenergic pathway is not involved in the unmasking process.
The effects of two agonists which mainly cause a selective activation of the atypical p3-adrenoreceg tars present in BAT are shown in Fig. 5. In warm adapted or warm re-exposed rats, injection of BRL
Depolarization (mV)
-so _
0
PRO PRO PRO
-SO,
5” 5’ NE NE IS0 Is0 PHE 5' 5' 5" NE NE NE
PRO PRO PRO PE PZ YO PE PZ YO
Fig. 4. Variations of membrane depolarization in brown adipose tissue mitochondria after acute injection of several a and /3 agonists (norepinephrine NE, phenylephrine PHE) and antagonists (propranolol PRO, pentholamine PE, prazozin PZ, yohimbine YO) to warm adapted rats (W.A.) and to 4-day warm re-exposed rats (after 10 days at 5°C) (W.R.). Antagonists were injected 20min before unmasking by injection of agonists (I hr at 28°C) or cold stress (I hr at 5°C). Six animals per groups; values are
mean & SEM.

Uncoupling protein in brown fat mitochondria 175
Depolarization (mv)
- so
0
W.A.
mm
W.R.
BRL BRL ICI ICI I
PRO PRO
BRL BRL
PRO
ml ICI ICI
PRO
Fig. 5. Effects of BRL 35135A and ICI D7114 injection (1 hr at 28°C) on variations of membrane depolarization in brown adipose tissue mitochondria of warm adapted rats (W.A.) and of 4-day warm re-exposed rats (after 10 days at 5°C) (W.R.). Six animals per groups; values are
mean f SEM.
35135A or ICI D7114 (300pg/kg i.m. 1 hr before sacrifice) led to about the same substantial membrane depolarization as ISO. These effects could not be counteracted by propranolol at a concentration high enough to inhibit NE stimulation.
DISCUSSION
The study of both GDP sensitive proton conduc- tance dependent on UCP over the whole range of membrane protonmotive force and membrane de- polarization clearly shows the great functional im- portance of unmasking and demonstrates how a b-adrenergic stimulation is involved in this process.
Based on UCP content and GDP binding measure- ments, unmasking of GDP binding sites is a well known phenomenon. However, the proton transloca- tion site is distinct from the regulatory nucleotide binding sites, and the few attempts to evaluate proton channelling by an indirect technique such as GDP dependent swelling in the presence of valinomycin have led to spreading results over one order of magnitude @wick and Swick, 1986; Trayhum et al., 1987; Peachey et al., 1988). Our direct approach indicates that cold stress at 5°C has a 4-fold promot- ing effect on CmH+ which is independent of the thermal history of the rat. However, warm re-exposed rats exhibit a higher potentiality for UCP unmasking than warm adapted rats as shown by the doubled effect of the potent /?-agonist isoproterenol.
Using Western blots, it has previously been esti- mated that there is 2.3 times more UCP in the mitochondria of warm re-exposed rats than those of warm adapted rats and that acute IS0 injection does not modify UCP content (Goubem et al., 1992). In the BAT of warm re-exposed rats, both the UCP content and the effective proton conductance are 80% of that previously observed in 10 day cold exposed rats where no masking was evidenced (Goubern et al., 1992); thus it is likely that injection
of the non specific b-agonist IS0 leads to a complete unmasking of the UCP proton channel naturally present in cold adapted rats. However, cold stress, which is the physiological stimulating factor, does not unmask UCP completely in warm re-exposed rats; it is likely that they have improved their cold tolerance and kept some potentiality for an increased response to a more severe cold stress (Heroux et al., 1977).
The ability of a B-adrenergic pathway to induce UCP unmasking is clearly demonstrated here. Com- parison of sympathetic control of unmasking process and UCP synthesis highlights two differences. Firstly, surgical denervation which completely impairs the unmasking process clearly demonstrates that the sympathetic nerves which directly innervate adipocytes play the major role in the regulation of acute activation of thermogenesis in cold stress. Other factors such as excessive epinephrine secretion by adrenal medulla during cold stress (Himms- Hagen, 1975) are unlikely to exert significant humoral stimulation under these conditions. At variance, sur- gical denervation did not completely prevent cold induced increase in mitochondrial UCP concen- tration, which was a slower process than unmasking (Park and Himms-Hagen, 1988). Secondly, UCP gene expression, both in rats in vivo and in brown adipocytes differentiated in culture, is mediated pri- marily by the action of norepinephrine on fl-adrener- gic receptors, although there is some evidence of a contribution via tl l-receptors (Jacobsson et al., 1986; Rehnmark et al., 1990). No such CI l-receptor partici- pation was found here in the unmasking process.
There is now substantial evidence from pharmaco- logical studies that adrenoreceptors are hetero- geneous in BAT (Arch, 1989; Girardier and Seydoux, 1986) and it is likely that the thermogenic response is mediated by a mixture of typical (presumably fi 1) and atypical (fl3) fi-adrenoreceptors (Feve et al., 1991). The b-agonist BRL, which selectively stimu- lates white and brown adipocytes in the rat, acts through atypical receptors (Arch et al., 1984; Hollenga et al., 1990; Holloway et al., 1991). Com- parison of the present work with the findings of Milner et al. (1988) would indicate substantial under- estimation in that study of the increase in UCP dependent CmH+ which accompanies the two-fold increase in GDP binding after acute treatment with BRL 26830A.
Atypical receptors are known to be poorly inhib- ited by the j-antagonist propranolol (Arch, 1989; Muzzin et al., 1992). The aspecific potent /?-agonist isoproterenol acts predominantly through /I 3 recep- tors (Feve et al., 1992), p l-receptors playing a small and subordinate role: the only limited inhibition by propranolol observed in this study agrees with this dominance. The lower affinity of the /33-receptor compared with that of classical p-receptors for nor- epinephrine agrees with the high level of inhibition of norepinephrine unmasking by propranolol observed here. This leads to the conclusion that in vivo injection
CBPC, 106,1-l.

176 M. Gouet 36-4 et al.
emorine L. J., Feve B., Pairault J., Briend-Sutren M. M., Marullo S., Delavier-Klutchko C. and Strosberg A. D. (1991) Structural basis for functional diversity of /?I-, fi2- and @3-adrenergic receptors. Biochem. Pharmac. 41, 853-859.
F&e B., emorine L. J., Lasnier F., Blin N., Baude B., Nahmias C., Strosberg A. D. and Pairault J. (1991) Atypical p-adrenergic receptor in 3T3-F442A adipocytes. J. biol. Chem. 266, 20329-20336.
Girardier L. and Seydoux J. (1986) Neural control of brown adipose tissue. In Brown Adipose Tissue (Edited by Tray- hum P. and Nicholls D. G.), pp. 214-268. Arnold, London.
Goubern M., Chapey M. F., Senault C., Laury M. C., Yazbeck J., Miroux B., Ricquier D. and Portet R. (1992) Effect of sympathetic de-activation on thermogenic func- tion and membrane lipid composition in mitochondria of brown adipose tissue. Biochim. biophys. Acta. 1107, 159-l 64.
Goubern M., Yazbeck J., Chapey M. F., Diolez P. and Moreau F. (1990) Variations in energization parameters and proton conductance induced by cold adaptation and essential fatty acid deficiency in mitochondria of brown adipose tissue in the rat. Biochim. biophys. Acta. 1015, 334-340.
Goubern M., Chapey M. F. and Portet R. (1991) Time- course variations of effective proton conductance and GDP binding in brown adipose tissue mit~hondria of rats during prolonged cold exposure. Camp. Physiot. Biochem. lOOB, 721-732.
Granneman J. G. and Lahners K. N. (1992) Differential adrenergic regulation of /I I- and fi3-adrenoreceptor mes- senger ribonucleic acids in adipose tissues. Endocrinology 130, 109-I 14.
Heroux O., Peter D. and Heggtveit A. (1977) Long-term effect of suboptimal dietary ma~esium on magnesium and calcium contents of organs, on cold tolerance and on lifespan and its pathological consequences in rats. J. Nurr. 107, 164&1652.
Himms-Hagen J. (1975) Role of the adrenal medulla in adaptation to cold. In Handbook ofPhysiology. Section I. Volume VI Adrenal gland., pp. 637-665. Am. Physiologi- cal Society, W~hington.
Himms-Hagen J. (1985) Brown adipose tissue metabolism and thermogenesis. Ann. Rev. Nutr. 5, 69-94.
Himms-Hagen J., Triandafillou J. and Gwilliam C. (1981) Brown adipose tissue of cafeteria fed rats. Am. J. Physiol. 241, El lbE120.
Hollenga Ch., Hass M., Deinen J. T. and Zaagsma J. (1990) Discrepancies in lipolitic activities induced by j%- adrenor~eptor agonists in human and rat adipocytes. Harm. metab. res. 22, 17-21.
Holloway B. R., Howe R., Rao B. S., Stribling D., Mayers R. M., Briscoe M. G. and Jackson J. M. (1991) ICI D7114 a novel selective /I-adrenoceptor agonist selectively stimu- lates brown fat and increases whole-body oxygen con- sumption. Br. 1. Pharmac. 104, 97-104.
Jacobsson A., Nedergaard J. and Cannon B. (1986) z- and ~-ad~nergic control of thermogenin mRNA ex- pression in brown adipose tissue. Bioscience reports 6, 621-631.
Kamo N., Muratsugu M., Hongoh R. and Kobatake Y. (1979) Membrane potential of mitochondria measured with an electrode sensitive to tetraphenyl phosphonium and relationship between proton electrochemical poten- tial and phosphorylation potential in steady state. J. membrane Biol. 81, 127-138.
Klaus S., Casteilla C., Bouillaud F. and Ricquier D. (1991) The uncoupling protein UCP: a membraneous mitochon- drial ion c&r&-exclusively expressed in brown adipose tissue. Int. J. Biochem. 23. 791-801. Derrv D. M.. Schonbaum E. and Steiner G. (19691 Two
sykpathetic nerves supplies to brown adipose fissuiof the Milner R. E., Wilson S., Aich J. R. C. and Trayhurn P. rat. Can. J. Physiot. Pharmac. 47, 57-63. (1988) Acute effects of a p-adrenoceptor agonist (BRL
of norepinephrine does not induce a sufficient concentration to act predominantly on P3-receptors. In cold stressed rats, a high level of norepinephrine is reached in BAT sympathetic synapses. From study of the thermal history of the rat, it is becoming clear that these two fl-adrenoreceptor subtypes exist, and the same function may be mediated in two somewhat different ways, depending upon the conditions of stimulation. In warm adapted rats it is likely that sympathetic stimulation acts preferentially through fi l-receptors, with propranolol inhibiting such stimu- lation. In contrast, in warm re-exposed rats, sympath- etic stimulation by cold stress is likely to involve atypical receptors, since no inhibitory effect of pro- pranolol was observed. One explanation could be that cold stress exerts a more intensive nervous BAT stimulation in the warm re-exposed rats than in the warm adapted ones leading to stimulation of both p l-receptors and less sensitive /J3-receptors in the synaptic cleft. However, a higher unmasking expected in the warm re-exposed rats has not been demonstrated.
One alternative possibity is that the relative num- ber of p-receptor subtypes implicated in the unmask- ing process may differ according to the thermal history of the rat, leading to a shift from p 1 to 83. Although neurotransmitter stimulation activates the different events leading to mitochondrial BAT heat production, growing evidence indicates that pro- longed exposure to high doses of catecholamines exerts a down regulation of /I3-receptors via changes in protein level (Holloway et al., 1991) and a marked desensitization of B l-receptors (Swartengren et al.,
1982), with the /33-adrenoreceptors not displaying desensitization sites (6morine ef al., 1991). Norepi- nephrine injection in warm re-exposed rats exerts a lesser effect which is compatible with a marked desensitization of /Y 1 -receptors; higher variability in this group may indicate variable desensitization. That possibility may explain the fact that propranolol may sometimes show an unexpectedly low potency for inhibition of the BAT fl-adrenergic the~ogenic re- sponse both in vivo and in vitro (Ricquier et al., 1984; Rothwell and Stock, 1984; Mohell et af., 1987).
REFERENCES
Arch J. R. C. (1989) The brown adipocyte /I?-adrenorecep- tor. Proc. Nutr. Sot. 48. 215-223.
Arch J. R. C., Ainsworth k. T., Cawthorne M. A., Piercy V.. Sennitt M. V.. Thodv V. E. and Wilson S. (1984) Atypical B-receptor on drown adipocytes as targkt fo; anti-obesity drugs. Nature 309, 163-165.
Cannon B. and Lindberg 0. (1979) Mitochondria from brown adipose tissue. Isolation and properties. Merh. Enzym. 60, 65-78.
Cottle M. K. W. and Cottle W. H. (1970) Adrenergic fibres in brown fat of cold acclimated rats. .I. Histochem. Cytochem. 18, 116-I 19.

Uncoupling protein in brown fat mitochondria 177
26830A) on rat brown adipose tissue mitochondria. Bio- them. J. 249, 759-763.
Mohell N., Connolly E. and Nedergaard J. (1987) Distinc- tion between the mechanisms underlying alpha, or B- adrenergic respiratory stimulation in brown fat cells. Am. J. Phvsiol. 253, C301X308.
Muzzin~ P., Revelli J. P., Fraser C. M., and Giacobino J. P. (1992) Radioliaand binding studies of the atypical ‘beta’ 3-adrenergic receptor ;n rat brown adi- pose tissue using (H-3)CPG12177. FEBS L.&r. 298, 162-164.
Nicholls D. G. (1982) Bioenergetics. Academic Press, London.
Nicholls D. G. and Locke R. M. (1984) Thermogenic mechanisms in brown fat. Physiol. Rev. 64, 164. _
Park I. R. A. and Himms-Hagen J. (1988) Neural influences on trophic changes in brown adipose tissue during cold acclimation. Am. J. Physiol. 255, R874R881.
Peachev T.. French R. R. and York D. A. (1988) Reeulation < ~ I
of GDP binding and uncoupling protein concentr&ion in brown adipose tissue mitochondria. Eiochem. J. 249, 451457.
Rehnmark S., Nechad M., Herron D., Cannon B. and Nedergaard J. (1990) a- and /3-adrenergic induction of the expression of the uncoupling protein thermogenin in brown adipocytes differentiated in culture. J. biol. Chem. 265,16464-16471.
Ricquier D. and Mory G. (1984) Factors affecting brown adipose tissue activity in animals and man. Clin. En- docrinol. Merab. 13, 501-520.
Ricquier D., Mory G., Bouillaud F., Thibault J. and Weissenbach J. (1984) Rapid increase of mitochondrial uncoupling protein and its mRNA in stimulated brown adinose tissue. FEBS L&r. 178. 24&244.
Rothwell N. J. and Stock M. J. (1984) Brown adipose tissue and diet induced thermogenesis. In Brown Adipose Tissue (Edited by Trayhurn P. and Nicholls D. G.), pp. 214268. Arnold, London.
Rottenberg H. (1984) Membrane potential and surface potential in mitochondria uptake and binding of lipophilic cations. Membrane Biol. 81, 127-138.
Shrago E. and Strieleman P. J. (1987)The biochemical mechanisms of brown fat thermogenesis. Wld. Rev. Nutr. Diet. 53, 171-217.
Smith R. E. (1961) Thermogenic activity of the hibernating gland of the cold acclimated rat. Physiologist 4, 113-l 14.
Swartengren J., Svoboda P. and Cannon B. (1982) Desensi- tisation of /3-adrenergic responsiveness in uiuo. Eur. J. Biochem. 128, 481-488.
Swick A. G. and Swick R. W. (1986) Rapid changes in number of GDP binding sites on brown adipose tissue mitochondria. Am. J. Physiol. 251, E192-E195.
Trayhum P., Aswell M., Jennings G., Richard D. and Stirling D. M. (1987) Effects of warm or cold exposure on GDP binding and uncoupling protein in rat brown fat. Am. J. Physiol. 252, E237-E243.
Trayhurn P. and Milner R. E. (1989) A commentary on the interpretation of in vitro biochemical measures of brown adipose tissue thermogenesis. Can. J. Physiol. Pharmac. 67, 81 l-819.