EEBΜΒ-2016-Poster-Meropi-Karakioulaki
-
Upload
meropi-karakioulaki -
Category
Documents
-
view
24 -
download
0
Transcript of EEBΜΒ-2016-Poster-Meropi-Karakioulaki

THE ROLE OF TRAF5 PROTEIN IN SIGNAL TRANSDUCTION BY THE EPSTEIN BARR VIRUS ONCOPROTEIN LMP1Meropi Karakioulaki, Paul Hadweh, George Mosialos
School of Biology, Aristotle University of Thessaloniki, 54124, Thessaloniki, Greece
AbstractThe Epstein-Barr virus (EBV) is a widespread human γ-herpes virus, which is capable of infecting epithelial cells as well as B-lymphocytes following either a lytic or a latent type of infection. The lytic infection during adolescence is associated with the clinical syndrome called infectious mononucleosis. The latent infection is associated with the development of various epithelial and lymphoid malignancies. The LMP1 protein is the major oncogenic protein of EBV and it is necessary for the transformation and immortalization of B-lymphocytes by the virus. Via its carboxyl terminal end the LMP1 protein interacts with various cytoplasmic factors and has the ability to mimic a constantly activated CD40 receptor and thus induce the transformation and uncontrolled proliferation of B-lymphocytes, mainly by activating the NF-κΒ transcription factor family. Among the cytoplasmic factors that are implicated in the LMP1 signal transduction are the TRAF protein family members. The least investigated among them is TRAF5, which seems to be essential for the viability of B-lymphocytes that have been infected by the EBV. In the present study we investigated the role of TRAF5 in the viability of epithelial cells that express LMP1 and depend on LMP1 for their survival. The results suggested that the repression of TRAF5 expression reduces the viability of these epithelial cells. This indicates that TRAF5 might be a potential target for the development of new therapeutic approaches against EBV-associated epithelial malignancies.
Downregulation of TRAF5 protein expression
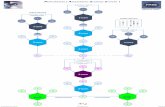
Figure 3. Clones 3 (LMP1+) and 9 were both transfected with an siRNA targeting the mRNA of the TRAF5 gene (siRNA TRAF5) or the luciferase gene (siRNA luc) as indicated. Whole cell lysates were subjected to immunoblotting with anti-LMP1, anti-TRAF5 and anti-β-actin antibodies. The TRAF5 protein expression was repressed by the siRNA TRAF5.
Figure 5. After transfecting both Clones 3 (LMP1+) and 9 with siRNA TRAF5 and siRNA luc, a cell viability assay using the MTT method was performed. As shown in the figure above, there was a statistically significant (p value= 0,041) the viability of Clone 3 (LMP1+), when the expression of the TRAF5 gene was repressed. Such a reduction was not apparent in Clone 9 (LMP1-) after the repression of TRAF5 gene expression.
ConclusionsTRAF5 protein plays a very important role for the viability of epithelial cells that express LMP1 and depend on LMP1 for their survival.TRAF5 might be a potential target for the development of new therapeutic approaches against EBV-associated epithelial malignancies that would not disrupt the
normal function of the CD40 receptor because TRAF5 doesn't appear to play an important role in the signal transduction of the CD40 receptor expressed in healthy cells (Kraus, Nakano, Bishop 2009 & Nakano et al. 1999).
Generation of HEK293FT cell lines that stably express LMP1 Figure 1. HEK293 cells were cotransfected with a plasmid that expresses the LMP1 gene (pcDNA3-LMP1) and a plasmid that contains the zeocin-resistance gene (pE-selectin-Zeo) under the control of the E-selectin promoter, which contains 3 NF-κΒ binding sites. Whole cell lysates of the transfected clones were subjected to immunoblotting with anti-LMP1 and anti-α-tubulin antibodies. Clone 3 stably expresses the LMP1 protein. Clone 9 was selected as a negative control.
This research was funded by the scholarship "Aristia 2015" funding postdoctoral researchers by ELKE AUTH.
Figure 4. Clones 3 and 9 were transfected with siRNA TRAF5 or siRNA luc and were subjected to RNA isolation and cDNA synthesis, followed by RT-PCR using primers that amplify the TRAF5 or GAPDH mRNAs. The figure shows the expression of TRAF5 mRNA in siRNA TRAF5-transfected cells relative to siRNA luc-transfected cells which was set at 100. Average values ±SE from 3 experiments are shown (p value for Clone 3= 0.011, p value for Clone 9= 0.01).
MTT Cell Viability Assay
Figure 2. Optical microscopy photos of Clone 9 (LMP1-, A: 10X, B: 20X) and Clone 3 (LMP1+, C: 10X, D: 20X). Due to the expression of the LMP1 protein in Clone 3, a significant difference in the shape and developmental pattern of the two clones can be observed.
Downregulation of TRAF5 mRNA by RNA interference