Python Poster (1)
Transcript of Python Poster (1)

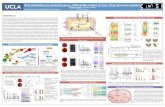
Hepatocyte Nuclear Factor 4α and Bile Acid Transport
Abstract
Introduction
Methods
Results
Conclusions
Acknowledgements References
Anne Cathryn Cox | | Python Project Fall 2016
Amplification
Standard Curve Melt Peak
❏ WGS sequence mapped to Gallus gallus annotated HNF4A gene
❏ Primers for HNF4A created from Whole Genome Shotgun (WGS) Python sequence
❏ Primers validated in-silico using NCBI BLAST
Primer Design RNA Isolation
❏ RNA isolated from fasted, 1, 3, and 10 day-post-fed python liver tissue using RNeasy column
❏ Two python specimens used per sample to minimize error due to individual python abnormalities
cDNA Synthesis❏ cDNA made from RNA using
(1) random hexamers, (2) oligo d(T), and (3) gene specific primers to find best synthesis route
❏ Gene specific primers found as best synthesis protocol
❏ cDNA synthesized for fasted, 1, 3, and 10 day-post-fed RNA (100% synthesis assumed)
Quantitative PCR❏ qPCR performed using SYBR
Select (binds to dsDNA) on diluted fasted, 1, 3, and 10 days-post-fed cDNA
❏ Standard curve created with known concentrations of pooled cDNA to interpolate starting quantity of unknowns
❏ Number of PCR cycles required to amplify was correlated to starting quantity
Primer Validation
❏ Products from qPCR reaction were run on a 1% agarose gel with Ethidium Bromide
❏ Product size verified as intended product from in-silico validation to verify that primers amplified correct gene
Bile Acid Transport and HNF4AHepatocyte nuclear factor 4 alpha (HNF4A) is one of several transcription factors that heavily regulate genes involving the transport of bile acids around the digestive system, largely from the liver where they are synthesized through the bile duct to the intestines to digest fats, and then back to the liver from the intestines. By focusing on a transcription factor for many transport proteins instead of one specific transport protein, expression levels can potentially be implied for many genes and not just one.
In knockout studies with mice, adult male mice had decreased levels of mRNA for NCTP (Sodium Taurocholrate Cotransporting Polypeptide) among others when HNF4A was rendered nonfunctional in the liver (Lu, Gonzalez, & Klaassen, 2010). NCTP, expressed less in the absence of HNF4A, is a transport pump and is the primary way in which bile acids recycle from the ileum and colon back into the hepatocyte for reuse. HNF4A has been shown to increase NCTP in other studies as well (Dietrich et al., 2007; Hayhurst, Lee, Lambert, Ward, & Gonzalez, 2001).
Python Bivittatus and Organ HypertrophyThe Burmese python has several key physiological processes that make it such a pertinent animal for research on human disease involving the metabolism of cholesterol and other fats, including metabolic syndrome, pathological cardiac hypertrophy, and hepatic steatosis (fatty liver disease).
The python’s digestive organs, including the heart, all grow in mass rapidly after consuming a meal (Secor, 2008). This organ growth is physiological and not pathological, and is attributed to a few select fatty acids present in the python’s serum that are not originally from the meal (Riquelme et al., 2011; Secor, 2008). Key insights from research on the Burmese python are already yielding new human implications for treating cardiac regression in cancer patients and cardiac hypertrophy (Riquelme et al., 2011). However, it is not well understood where these key fatty acids come from in the python serum, and how the python processes the large amount of fat in its postprandial system and serum. In order to address this, researchers from the Python Project under the lab of Dr. Leslie Leinwand are looking into specific genes that are known in humans to play a role in either the synthesis or transport of bile acids, which emulsify fats in the digestive system and allow them to be processed. By unlocking the key to how pythons process such large amounts of fat and remain healthy, human conditions such as metabolic syndrome can be looked at from another, perhaps more enlightening angle than previous research.
Figure from (Secor 2008)
Figure from (Riquelme et al., 2011)
In another knockout study, the serum chemistry of wild-type vs HNF4A-null mice was analyzed (Hayhurst et al., 2001). This study found that in addition to having decreased NCTP levels by western blot, HNF4A null mice had increased levels of ALT, triglycerides, total cholesterol, HDL cholesterol, and bile acids in their serum (Hayhurst et al., 2001). This leads to the conclusion that HNF4A must be important to getting bile acids out of an organism’s serum and into its hepatocytes for use in the liver.
Another important function of HNF4A is to help hepatocyte nuclear factor 1 alpha (HNF1A), another important hepatic transcription factor, to bind its promoters on several other hepatic genes in liver cell nuclei (Eeckhoute, Formstecher, & Laine, 2004). When it does this, the genes which are activated by HNF1A, such as Cholesterol 7-Alpha-Hydroxylase (CYP7A1) are also upregulated. CYP7A1 plays a vital role and is the rate determining step in the conversion of cholesterol into bile acids.
Because of HNF4A’s varying functions in bile acid synthesis and export, it may hold key insights into fat digestion in the Burmese python.
Figure from (Dawson et al., 2009)
hepatocyte nuclear factor 4 alpha (HNF4A) expression in python liver tissue was tested using real-time PCR. HNF4A has two separate functions important to bile acid homeostasis in hepatocytes in humans: (1) to upregulate the transcription of transport proteins (largely NCTP) moving recycled bile acid into hepatic cells while downregulating transport proteins which move bile acids from hepatic cells into the canaliculus, and (2) to increase binding of HNF1A to its promoter thereby increasing the transcription of CYP7A1 and the downstream synthesis of bile acids. This research found that expression of HNF4A mRNA doubles from the fasted tissue at 1 day-post-fed (DPF), and then returns to normal by 3 DPF, suggesting that the python uses similar pathways of bile-acid sequestration in hepatocytes as humans.
Figure from (Riquelme et al., 2011)
Figure from (Halilbasic et al., 2013)
Over 1 in 5 adults in the US has metabolic syndrome (MetS), which is a collection of symptoms including some or all of the following: obesity, high fasting plasma glucose, high blood pressure, high triglyceride levels, and low HDL cholesterol levels (Beltrán-Sánchez, Harhay, Harhay, & McElligott, 2013). Although this syndrome is well studied, there is still much to learn about how humans process fat in the liver. The Burmese python has several key physiological processes that make it such a pertinent animal for research on human disease involving the metabolism of cholesterol and other fats, including MetS, pathological cardiac hypertrophy, and hepatic steatosis (fatty liver disease). One of these processes is the rapid metabolism of serum fatty acids post-meal. To explore this process,
Primer DesignIn order to find successful primers that would amplify only the intended gene product, the
Real Time PCR
Gallus gallus annotated genome sequence of HNF4A was blasted against the python whole genome shotgun from NCBI, which produced the results shown here. The majority of the gene was able to be assembled using the corresponding contigs from the python WGS, mapping to the order of the Gallus gallus transcript.
The primers shown were chosen because they had acceptable length, melt temperature for real time PCR (~60°C), low G-C content, and low self-complementarity. Additionally, they were in 2 different exons, which helps to distinguish mRNA from genomic contamination in the real time PCR experiment.
As an additional precaution, the three possible reading frames of the assembled putative python transcript were translated online with ExPASy to verify that an open reading frame existed.
Standards (Known starting concentrations of
gene-specific cDNA to generate standard curve)
10 ng/µL1 ng/µL
0.1 ng/µL0.01 ng/µL
Pure water
Fasted1 day post fed3 days post fed10 days post fed
Unknowns(cDNA synthesized from python liver tissue at different time point after eating)
To measure changes in gene expression levels of HNF4A, gene specific cDNA from fasted, 1 dpf, 3 dpf, and 10 dpf was synthesized from pooled RNA from two different pythons using the designed primers. This cDNA was then used to set up a real time PCR plate as shown below, using known concentrations of pooled cDNA in order to create a standard curve to interpolate the starting cDNA concentrations of the unknown samples.
The data show that HNF4A is upregulated by approximately 2 fold one day after the python has had a meal. Additionally,
HNF4A remains slightly upregulated at 3 and 10 days post fed.
In subsequent experiments, the expression of NCTP could be measured using the same methodology used in this study. This would provide insights into how important NCTP is to bile acid homeostasis. Additionally, other bile acid transporters such as ASBT, OSTa/OSTb, and BSEP could be explored. Knowing the changes in expression for these genes would allow a more complete picture of bile acid transport in the postprandial Burmese python. Finally, HNF1A (hepatocyte nuclear factor 1 alpha) could be measured, to solidify the relationship between HNF4A and HNF1A.
DN
A L
ad
der
qP
CR
Pro
du
ct
qP
CR
Pro
du
ct
Co
ntr
ol
wel
l
100 bp200 bp
Product size from primer
design: 121 bp
The products of the qPCR reaction were run on an agarose gel in order to verify intended product size (i.e. rule out another product as the cause of amplification). To the left, the gel obtained from this experiment is shown. The qPCR product lanes show a band between 100 bp and 200 bp on the DNA ladder, which represents the designed product between the primers of 121 bp. Additionally, the control well showed no bands, indicating little contamination.
Product Validation
The standard curve above shows the relationship between the cycle of qPCR which a product began to amplify and the
starting quantity of that product. It was prepared using known dilutions of cDNA.
After the PCR cycles, the qPCR machine performed a melt test to show the number of distinct products produced in the qPCR
reaction. Since only one peak with the same temperature is above the threshold line, only one product was amplified.
The graph above amplification measured by fluorescence of a dye which fluoresces when bound to dsDNA. Each curve
represents one well on the qPCR plate. The product which amplifies with less cycles had a higher starting concentration.
The most important results of the qPCR experiment are shown above. The starting concentration of HNF4A cDNA in the fasted, 1
dpf, 3 dpf, and 10 dpf python liver samples were interpolated from the standard curve and amplification data obtained by qPCR.
Figure from (Secor 2008)
● HNF4A plays an important role in the digestive physiology of the Burmese python, and that role is similar to the role that has been demonstrated in humans.
● HNF4A is upregulated at 1 dpf, when the highest concentration of lipids is present in the burmese python serum, and before the majority of its meal has been digested. It is likely that NCTP is also upregulated at this time.
● It is possible that in the python, HNF4A sequesters bile acids in the hepatocytes in preparation of releasing them to digest the meal. This sequestration could explain the liver hypertrophy seen early after the snake has fed but before it has digested its meal.
Future Experiments
Battle, Michele A., Genevieve Konopka, Fereshteh Parviz, Alexandra Lerch Gaggl, Chuhu Yang, Frances M. Sladek, and Stephen A. Duncan. “Hepatocyte Nuclear Factor 4α Orchestrates Expression of Cell Adhesion Proteins during the Epithelial Transformation of the Developing Liver.” Proceedings of the National Academy of Sciences 103, no. 22 (May 30, 2006): 8419–24. doi:10.1073/pnas.0600246103.
Beltrán-Sánchez, Hiram, Michael O. Harhay, Meera M. Harhay, and Sean McElligott. “Prevalence and Trends of Metabolic Syndrome in the Adult U.S. Population, 1999–2010.” Journal of the American College of Cardiology 62, no. 8 (August 20, 2013): 697–703. doi:10.1016/j.jacc.2013.05.064.
Bonzo, Jessica A., Christina H. Ferry, Tsutomu Matsubara, Jung-Hwan Kim, and Frank J. Gonzalez. “Suppression of Hepatocyte Proliferation by Hepatocyte Nuclear Factor 4α in Adult Mice.” The Journal of Biological Chemistry 287, no. 10 (March 2, 2012): 7345–56. doi:10.1074/jbc.M111.334599.
Chen, Jean, Allen D. Cooper, and Beatriz Levy-Wilson. “Hepatocyte Nuclear Factor 1 Binds to and Transactivates the Human but Not the Rat CYP7A1 Promoter.” Biochemical and Biophysical Research Communications 260, no. 3 (July 1999): 829–34. doi:10.1006/bbrc.1999.0980.
Dawson, Paul A., Tian Lan, and Anuradha Rao. “Bile Acid Transporters.” Journal of Lipid Research 50, no. 12 (December 2009): 2340–57. doi:10.1194/jlr.R900012-JLR200.Dietrich, Christoph G., Ina V. Martin, Anne C. Porn, Sebastian Voigt, Carsten Gartung, Christian Trautwein, and Andreas Geier. “Fasting Induces Basolateral Uptake Transporters of the SLC
Family in the Liver via HNF4alpha and PGC1alpha.” American Journal of Physiology. Gastrointestinal and Liver Physiology 293, no. 3 (September 2007): G585-590. doi:10.1152/ajpgi.00175.2007.
Eeckhoute, J., P. Formstecher, and B. Laine. “Hepatocyte Nuclear Factor 4α Enhances the Hepatocyte Nuclear Factor 1α-Mediated Activation of Transcription.” Nucleic Acids Research 32, no. 8 (2004): 2586–93. doi:10.1093/nar/gkh581.
Ekholm, E., R. Nilsson, L. Groop, and C. Pramfalk. “Alterations in Bile Acid Synthesis in Carriers of Hepatocyte Nuclear Factor 1α Mutations.” Journal of Internal Medicine 274, no. 3 (September 1, 2013): 263–72. doi:10.1111/joim.12082.
Gbaguidi, G. Franck, and Luis B. Agellon. “The Inhibition of the Human Cholesterol 7alpha-Hydroxylase Gene (CYP7A1) Promoter by Fibrates in Cultured Cells Is Mediated via the Liver X Receptor Alpha and Peroxisome Proliferator-Activated Receptor Alpha Heterodimer.” Nucleic Acids Research 32, no. 3 (2004): 1113–21. doi:10.1093/nar/gkh260.
Halilbasic, Emina, Thierry Claudel, and Michael Trauner. “Bile Acid Transporters and Regulatory Nuclear Receptors in the Liver and beyond.” Journal of Hepatology 58, no. 1 (January 2013): 155–68. doi:10.1016/j.jhep.2012.08.002.
Hayhurst, Graham P., Ying-Hue Lee, Gilles Lambert, Jerrold M. Ward, and Frank J. Gonzalez. “Hepatocyte Nuclear Factor 4α (Nuclear Receptor 2A1) Is Essential for Maintenance of Hepatic Gene Expression and Lipid Homeostasis.” Molecular and Cellular Biology 21, no. 4 (February 2001): 1393–1403. doi:10.1128/MCB.21.4.1393-1403.2001.
Lu, Hong, Frank J. Gonzalez, and Curtis Klaassen. “Alterations in Hepatic mRNA Expression of Phase II Enzymes and Xenobiotic Transporters after Targeted Disruption of Hepatocyte Nuclear Factor 4 Alpha.” Toxicological Sciences: An Official Journal of the Society of Toxicology 118, no. 2 (December 2010): 380–90. doi:10.1093/toxsci/kfq280.
Pauli-Magnus, Christiane, Bruno Stieger, Yvonne Meier, Gerd A. Kullak-Ublick, and Peter J. Meier. “Enterohepatic Transport of Bile Salts and Genetics of Cholestasis.” Journal of Hepatology 43, no. 2 (August 2005): 342–57. doi:10.1016/j.jhep.2005.03.017.
Riquelme, Cecilia A., Jason A. Magida, Brooke C. Harrison, Christopher E. Wall, Thomas G. Marr, Stephen M. Secor, and Leslie A. Leinwand. “Fatty Acids Identified in the Burmese Python Promote Beneficial Cardiac Growth.” Science (New York, N.Y.) 334, no. 6055 (October 28, 2011): 528–31. doi:10.1126/science.1210558.
Secor, Stephen M. “Digestive Physiology of the Burmese Python: Broad Regulation of Integrated Performance.” Journal of Experimental Biology 211, no. 24 (December 15, 2008): 3767–74. doi:10.1242/jeb.023754.
Servitja, Joan-Marc, Miguel Pignatelli, Miguel Angel Maestro, Carina Cardalda, Sylvia F. Boj, Juanjo Lozano, Enrique Blanco, et al. “Hnf1alpha (MODY3) Controls Tissue-Specific Transcriptional Programs and Exerts Opposed Effects on Cell Growth in Pancreatic Islets and Liver.” Molecular and Cellular Biology 29, no. 11 (June 2009): 2945–59. doi:10.1128/MCB.01389-08.
Thomas, Charles, Roberto Pellicciari, Mark Pruzanski, Johan Auwerx, and Kristina Schoonjans. “Targeting Bile-Acid Signalling for Metabolic Diseases.” Nature Reviews Drug Discovery 7, no. 8 (August 2008): 678–93. doi:10.1038/nrd2619.
Yahoo, Neda, Behshad Pournasr, Jalal Rostamzadeh, and Fardin Fathi. “Forced Expression of Hnf4a Induces Hepatic Gene Activation through Directed Differentiation.” Biochemical and Biophysical Research Communications 476, no. 4 (August 5, 2016): 313–18. doi:10.1016/j.bbrc.2016.05.119.
Beltrán-Sánchez, H., Harhay, M. O., Harhay, M. M., & McElligott, S. (2013). Prevalence and Trends of Metabolic Syndrome in the Adult U.S. Population, 1999–2010. Journal of the American College of Cardiology, 62(8), 697–703. https://doi.org/10.1016/j.jacc.2013.05.064
Dawson, P. A., Lan, T., & Rao, A. (2009). Bile acid transporters. Journal of Lipid Research, 50(12), 2340–2357. https://doi.org/10.1194/jlr.R900012-JLR200
Dietrich, C. G., Martin, I. V., Porn, A. C., Voigt, S., Gartung, C., Trautwein, C., & Geier, A. (2007). Fasting induces basolateral uptake transporters of the SLC family in the liver via HNF4alpha and PGC1alpha. American Journal of Physiology. Gastrointestinal and Liver Physiology, 293(3), G585-590. https://doi.org/10.1152/ajpgi.00175.2007
Eeckhoute, J., Formstecher, P., & Laine, B. (2004). Hepatocyte Nuclear Factor 4α enhances the Hepatocyte Nuclear Factor 1α-mediated activation of transcription. Nucleic Acids Research, 32(8), 2586–2593. https://doi.org/10.1093/nar/gkh581
Halilbasic, E., Claudel, T., & Trauner, M. (2013). Bile acid transporters and regulatory nuclear receptors in the liver and beyond. Journal of Hepatology, 58(1), 155–168. https://doi.org/10.1016/j.jhep.2012.08.002
Hayhurst, G. P., Lee, Y.-H., Lambert, G., Ward, J. M., & Gonzalez, F. J. (2001). Hepatocyte Nuclear Factor 4α (Nuclear Receptor 2A1) Is Essential for Maintenance of Hepatic Gene Expression and Lipid Homeostasis. Molecular and Cellular Biology, 21(4), 1393–1403. https://doi.org/10.1128/MCB.21.4.1393-1403.2001
Lu, H., Gonzalez, F. J., & Klaassen, C. (2010). Alterations in hepatic mRNA expression of phase II enzymes and xenobiotic transporters after targeted disruption of hepatocyte nuclear factor 4 alpha. Toxicological Sciences: An Official Journal of the Society of Toxicology, 118(2), 380–390. https://doi.org/10.1093/toxsci/kfq280
Riquelme, C. A., Magida, J. A., Harrison, B. C., Wall, C. E., Marr, T. G., Secor, S. M., & Leinwand, L. A. (2011). Fatty Acids Identified in the Burmese Python Promote Beneficial Cardiac Growth. Science (New York, N.Y.), 334(6055), 528–531. https://doi.org/10.1126/science.1210558
Secor, S. M. (2008). Digestive physiology of the Burmese python: broad regulation of integrated performance. Journal of Experimental Biology, 211(24), 3767–3774. https://doi.org/10.1242/jeb.023754
Thanks to Dr. Leslie Leinwand and Dr. Pamela Harvey for continuing to make the Python Project a
worthwhile and genuine undergraduate research experience.