Direct, Pyrrolidine Sulfonamide Promoted Enantioselective Aldol Reactions of α,α-Dialkyl...
1
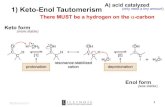
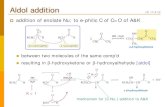
2005 Alcohols Q 0230 Direct, Pyrrolidine Sulfonamide Promoted Enantioselective Aldol Reactions of α,α-Dialkyl Aldehydes: Synthesis of Quaternary Carbon-Containing β-Hydroxy Carbonyl Compounds. — A bifunctional (S)-pyrrolidine trifluoromethanesulfon- amide is an effective organocatalyst for the direct asymmetric aldol reaction of α,α-dialkyl aldehydes. β-Hydroxyaldehydes with an α-quaternary center are obtained. — (WANG*, W.; LI, H.; WANG, J.; Tetrahedron Lett. 46 (2005) 30, 5077-5079; Dep. Chem., Univ. N. Mex., Albuquerque, NM 87131, USA; Eng.) — Mais 44- 092
Transcript of Direct, Pyrrolidine Sulfonamide Promoted Enantioselective Aldol Reactions of α,α-Dialkyl...
2005
AlcoholsQ 0230 Direct, Pyrrolidine Sulfonamide Promoted Enantioselective Aldol Reactions of
α,α-Dialkyl Aldehydes: Synthesis of Quaternary Carbon-Containing β-Hydroxy Carbonyl Compounds. — A bifunctional (S)-pyrrolidine trifluoromethanesulfon-amide is an effective organocatalyst for the direct asymmetric aldol reaction ofα,α-dialkyl aldehydes. β-Hydroxyaldehydes with an α-quaternary center are obtained. — (WANG*, W.; LI, H.; WANG, J.; Tetrahedron Lett. 46 (2005) 30, 5077-5079; Dep. Chem., Univ. N. Mex., Albuquerque, NM 87131, USA; Eng.) — Mais
44- 092