Copper ligation to soluble oligomers of the English mutant of the amyloid-β peptide yields a linear...
Transcript of Copper ligation to soluble oligomers of the English mutant of the amyloid-β peptide yields a linear...
This journal is c The Royal Society of Chemistry 2013 Chem. Commun., 2013, 49, 4797--4799 4797
Cite this: Chem. Commun.,2013,49, 4797
Copper ligation to soluble oligomers of the Englishmutant of the amyloid-b peptide yields a linear Cu(I)site that is resistant to O2 oxidation†
Kristy L. Peck, Heather S. Clewett, Jennifer C. Schmitt and Jason Shearer*
Copper coordination to soluble oligomers of the English (AbH(6)R)
mutant of the amyloid-b peptide is probed. Cu(II) coordination
yields a square planar (N/O)4 coordination environment, while
reduction yields an O2 inert linear bis-His Cu(I) centre.
Alzheimer’s disease (AD) is a fatal neurodegenerative disorder that iscurrently the most common cause of senile dementia.1 It appearsthat a key step in AD is the formation of amyloid-b (Ab) peptidesfrom the processing of the neuronal membrane amyloid precursorprotein (APP), a ubiquitous protein of unknown function.2 Depend-ing on the manner in-which the APP is cleaved different Ab peptidescan be produced, of which Ab(42) (DAEFR HDSGY EVHHQ KLVFFAEDVG SNKGA IIGLM VGGVV IA) appears to be the most neuro-toxic. Once formed, Ab(42) monomers tend to aggregate rapidlyforming soluble low molecular weight oligomers, which subse-quently form insoluble proteinaceous plaques; all three of theseforms have a high affinity for copper ions.
Although the proteinaceous plaques are a hallmark of AD, itappears that the soluble oligomers are the most neurotoxic form ofAb(42).3 Isolated Ab-dimers from the human brain have been shownto be synaptotoxic, as are synthetically prepared Ab-dimers.4,5 How-ever, in a recent study it was shown that freshly prepared syntheticAb-dimers are not highly synaptotoxic requiring an incubation timeto become toxic.6 This suggests that Ab-dimers may be templates forthe formation of larger soluble neurotoxic Ab-oligomers.
AD is mostly thought of as a geriatric disorder, however there isa small but significant number of individuals who begin display-ing symptoms of AD in their early 40s.7 Early onset AD has beenlinked to a number of different mutations of the APP and thesubsequent Ab peptides. One of the mutants that has been linkedto early on set AD is the so-called English mutant (Ab(42)H(6)R).8
Herein we explore the Cu(II) and Cu(I) coordination environmentsof soluble Ab(42)H(6)R oligomers. It will be shown that the Cu(I)coordination environment and redox properties of Ab(42)H(6)R
soluble oligomers at physiological pH are altered from wild-typeAb(42) soluble oligomers.9
Soluble non-fibril preglobulamers (soluble oligomers) ofAb(42)H(6)R peptides (30 kDa) stabilised by a small concentrationof SDS were prepared according to previously described protocols.10,11
This allows for the generation of a well defined oligomeric stateto probe potentially biologically relevant coordination chemistries.Preparation and handling of the soluble oligomers of Ab(42)H(6)Rpeptides are more difficult than wild type Ab(42) peptides due totheir propensity to form insoluble fibrils. However, we canreadily prepare both copper-coordinated and metal-free solubleoligomers of Ab(42)H(6)R oligomers in concentrations of at least300 mM as long as the samples are maintained at low tempera-ture (2 1C) and not excessively agitated. This was confirmed byGPC, FT-IR and Thioflavin-T dye-binding assays.11,12
Cu(II) coordination to soluble oligomers of Ab(42)H(6)R peptideswas first probed by X-band EPR spectroscopy (Fig. 1). Previously itwas shown that soluble oligomers of wild-type {CuII(Ab(42))} consistof one major species in solution with gJ = 2.274 (AJ = 169(3) �10�4 cm�1).9 This was tentatively identified as a 4-coordinateCu(II)-His3(N/O) centre. The EPR spectrum of {CuII(Ab(42)H(6)R)}oligomers can be contrasted to this; spectral deconvolution yieldstwo distinct Cu(II) coordination environments.11 The first cooper site(component I) displays a gJ = 2.260(2) (AJ = 163(2)� 10�4 cm�1) and
Fig. 1 X-band EPR spectrum of {CuII(Ab(42)H(6)R)} soluble oligomers (solid line)in 1 : 1 50 mM NEM buffer (pH 7.4) : glycerol (85 K, n = 9.11 GHz, amplitudemodulation = 5.0 G, microwave power = 20 mW). The dashed line isthe simulated spectrum (Component I: gJ = 2.260, g> = 2.040, AJ = 163 �10�4 cm�1, A> = 9 � 10�4 cm�1; Component II: gJ = 2.216, g> = 2.039,AJ = 151 � 10�4 cm�1, A> = 6 � 10�4 cm�1; I : II = 1.00 : 1.5114).11
Department of Chemistry, University of Nevada, Reno, NV 89557, USA.
E-mail: [email protected]
† Electronic supplementary information (ESI) available. See DOI: 10.1039/c3cc40326b
Received 15th January 2013,Accepted 15th April 2013
DOI: 10.1039/c3cc40326b
www.rsc.org/chemcomm
ChemComm
COMMUNICATION
Publ
ishe
d on
23
Apr
il 20
13. D
ownl
oade
d by
Hei
nric
h H
eine
Uni
vers
ity o
f D
uess
eldo
rf o
n 25
/09/
2013
11:
24:1
4.
View Article OnlineView Journal | View Issue
4798 Chem. Commun., 2013, 49, 4797--4799 This journal is c The Royal Society of Chemistry 2013
the second copper site has a gJ = 2.216(2) (AJ = 151(2) � 10�4 cm�1).Deconvolution yields a component I : II ratio of 1 : 1.5. As thecomponents are in equilibrium different ratios of components I : IImay be observed under slightly different sample conditions.
Based on modified EPR ‘‘truth tables’’ of Peisach andBlumberg,11,13 and Cu(II)-Ab studies we suggest that componentI (gJ = 2.260(2), AJ = 163(2) � 10�4 cm�1) is consistent with aCu(II)His2(N/O)2 centre, while component II (gJ = 2.216(2), AJ =151(2) � 10�4 cm�1) is consistent with a Cu(II)HisN�2(N/O)coordination environment (Scheme 1). Caution should betaken in considering the above EPR based structural assign-ments as they are based on empirical structure/spectroscopiccorrelations. EPR studies by Hureau and coworkers on mono-meric {CuIIAb(16)H(6)R}, which contains the copper bindingN-terminus but does not undergo aggregation, displays twocomponents (gJ = 2.26, AJ = 180 � 10�4 cm�1 and gJ = 2.22,AJ = 162 � 10�4 cm�1).14 These were assigned asCuIIHis2OcarbonylNamine and CuIIHisOcarbonylNamideNamine coordina-tion motifs. Thus, the Cu(II) coordination environment betweenAb(42)H(6)R monomers and oligomers may be distinct.
The Cu(II) centre can be effectively reduced by the addition of 1 eq.of ascorbate at room temperature as noted by the complete disap-pearance of the EPR signal. Previously, Cu(II) reduction to a coordi-nated Cu(I) ion in wild-type {CuII(Ab(42))} soluble oligomers andmonomers was noted. In the case of wild-type oligomers a highlyO2 reactive tetrahedral Cu(I) species is formed that is rapidly oxidizedback to {CuII(Ab(42))}.9 In contrast, the Cu(I) centres of reduced{CuI(Ab(42)H(6)R)} soluble oligomers are not oxidized upon O2 expo-sure to {CuII(Ab(42)H(6)R)}. This is reminiscent of how monomers of{CuI(Ab)} behave.15 In the case of {CuI(Ab)} monomers a linear bis-HisCu(I) coordination environment is formed,11 which is reasonably O2
stable taking B20 hours to reoxidize back to {CuII(Ab)} with subse-quent peptide-based oxidative damage noted by HPLC.15c The solubleoligomers of {CuII(Ab(42)H(6)R)} are especially resistant to O2 inducedoxidation; EPR spectroscopy shows no signal for {CuII(Ab(42)H(6)R)},even after 48 hours of air exposure, and HPLC shows no peptide-based modification events.11 We note that the above studies ignorepossible ternary complexes formed between {CuII(Ab(42)H(6)R)}and exogenous ligands found in the extracellular fluid, whichmay be important in AD.
The Cu K-edge X-ray absorption spectra of Cu(I) and Cu(II)coordinated oligomers of {Cu(Ab(42)H(6)R)} can be contrasted tothose of soluble oligomers of {Cu(Ab(42))}.9 Unlike the single welldefined 4-coordinate His3(N/O) Cu(II) structure of {CuII(Ab(42))}soluble oligomers, the Cu(II) coordination environment of{CuII(Ab(42)H(6)R)} is consistent with a mixture of four coordi-nate Cu(II)-coordination motifs. The data was best modelledwith 1 imidazole scatterers in one shell, 0.5 imidazole scatterersin a second and 2.5 N/O scatterers in a third shell, suggestive of
a mixed coordination environment as noted by EPR. Reductionof the Cu(II) centre to Cu(I) yields a linear two coordinate Cu(I)centre as evidenced by the XANES region of the X-ray absorp-tion spectrum; there is a prominent peak at 8984.2(1) eV in thepre-edge region of the XANES of soluble oligomers of{CuI(Ab(42)H(6)R)} (Fig. 2), which is characteristic of lineartwo-coordinate Cu(I).16 Analysis of the EXAFS region is alsoconsistent with a linear Cu(I) centre. The best fit to the dataindicates a His-CuI-His motif with a N–Cu–N bond angle of175(5)1 (j) and Cu–N bond lengths of 1.88 Å.11 This is con-sistent with what was previously observed with {CuI(Ab)} mono-mers. We note that prolonged O2 exposure (36 hours) yields nonoticeable change in the X-ray absorption spectrum. It there-fore appears that the linear Cu(I) centre in {CuI(Ab(42)H(6)R)}soluble oligomers is especially inert towards O2 oxidationevents (Scheme 1).
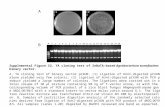
To gain insight into the differential coordination environ-ment and reactivity of {CuI(Ab(42)H(6)R)} vs. {CuI(Ab(42))}soluble oligomers we employed molecular dynamics (MD)simulations of Ab(42)H(6)R vs. Ab(42) hexamers.11 The hexamericform of Ab was chosen as these are of a similar molecular weightas the soluble oligomers. MD simulations were performed at310 K over a period of 350 ns. For wild-type Ab(42) hexamersequilibrium energies were reached within B40 ns, while equili-brium energies were reached within B55 ns for Ab(42)H(6)R.Examination of equilibrium structures demonstrate a cleardifference between the two hexameric structures (Fig. 3). Con-sistent with experimental data,17 the wild-type Ab(42) hexamerdisplays high b-sheet content that is well ordered. The b-sheetstructure persists from the C-terminus towards the copper-binding N-terminus (for the sake of this discussion we willdefine the N-terminus as being between residues 1–18); theb-sheets extend well into the N-terminal region ending betweenY(10)–S(8) depending on whether the Ab peptide is an interiorof exterior peptide. Over the course of the simulations little
Scheme 1 Reduction of {CuII(Ab(42)H(6)R)} to {CuI(Ab(42)H(6)R)}. The orienta-tion of the ligands about the Cu(II) centres are not meant to indicate the absoluteligand stereochemistry.
Fig. 2 Cu K-edge X-ray absorption spectrum of {CuI(Ab(42)H(6)R)} displaying (top)the XANES region, (bottom) the magnitude FT-EXAFS (FT from k = 2.0–12.0 Å�1) and(inset) the FF-EXAFS (back-transformed from r = 1.0–4.0 Å). For the EXAFS plots,the solid line represent the experimental data, the thick dashed line representsthe modelled data and the thin dotted line are the difference spectra. Cu-His2
shell: n = 1; r = 1.882(6) Å; s2 = 0.0031(4) Å2; j = 175(5)1; y = 32.6(22)1;Eo = 8985.4(2) eV; GOF = 0.37.11
Communication ChemComm
Publ
ishe
d on
23
Apr
il 20
13. D
ownl
oade
d by
Hei
nric
h H
eine
Uni
vers
ity o
f D
uess
eldo
rf o
n 25
/09/
2013
11:
24:1
4.
View Article Online
This journal is c The Royal Society of Chemistry 2013 Chem. Commun., 2013, 49, 4797--4799 4799
change in the structure was observed and the snapshoot inFig. 3 remains virtually static. We note that the wild-typehexamer is dramatically different from how wild-type monomersbehave, which appears to form rapidly interchanging a-helix/coilstructures.18
This can be contrasted with the Ab(42)H(6)R mutant. Althoughthis possess a high b-sheet content, there is far more flexibility in theN-terminal domain. Going towards the N-terminus, the b-sheet endsat approximately residue Q(15). Beyond Q(15) the N-terminusbehaves similarly to how the N-terminus behaves in Ab monomers;the N-terminus displays rapidly interconverting coil/helical struc-tures. This is due primarily to the large degree of electrostaticrepulsion between R(5) and R(6) on opposing Ab(42)H(6)R strands.Absent are the R(5) and H(6) H-bonding interactions on opposingAb(42) strands that stabilises the wild-type hexamer. One mighttherefore expect that the Ab(42)H(6)R hexamer will coordinate Cu(I)like a monomer, which is what is observed.
In summary, we have demonstrated the differential coppercoordination environment of Ab(42)H(6)R SDS stabilised solubleoligomers vs. Ab(42) SDS stabilised soluble oligomers. Most impor-tantly, a redox inactive linear bis-His Cu(I) coordination environmentis found in soluble oligomers of {CuI(Ab(42)H(6)R)}, which isidentical to what is observed in monomers of {CuIAb(42)}. This islikely due to a more ‘‘monomer-like’’ oligomeric N-terminus inhexamers of Ab(42)H(6)R vs. Ab(42). It is also possible that the lackof H(6) as a ligand may be altering the Cu(I) coordination environ-ment in the Ab(42)H(6)R vs. Ab(42) oligomers.
Some have assumed that redox active copper-bound Ab isresponsible for oxidative stress and subsequent neuronal damagein the O2 rich reducing environment of the cerebral spinal fluid.19
These data on the Ab(42)H(6)R mutant, which promotes earlyonset AD, demonstrates that oligomers of {CuI(Ab(42)H(6)R)} areboth inert towards O2 and cannot induce oxidative damage. Theimplication is that either copper-Ab based O2/reductant redox
cycling is not responsible for the initial stages of AD, or thatmultiple AD mechanisms are occurring. For example, consideringthe high affinity of Cu(I) for Ab monomers20 it could be that Aboligomers are disrupting cellular copper homeostasis. In addi-tion to biological studies, further detailed investigations of thefundamental coordination chemistries of these peptides areneeded to sort out such issues.
This work was supported by the NSF (CHE-0844234 andCHE-0840429). Beamtime for XAS measurements at the NSLSwas supported by the DOE (contract no. DE-AC02-98CH10886)and NIH (P30-EB-009998). We thank Dr Veronika Szalai(National Institutes of Standards and Technology, GaithersburgMD, USA) for valuable advice.
Notes and References1 C. P. Ferri, M. Prince, C. Brayne, H. Brodaty, L. Fratiglioni,
M. Ganguli, K. Hall, K. Hasegawa, H. Hendrie, Y. Huang, A. Jorm,C. Mathers, P. R. Menezes, E. Rimmer and M. Scazufca, Lancet, 2005,35, 352.
2 D. M. Holtzman, J. C. Morris and A. M. Goate, Sci. Transl. Med.,2011, 3, 77sr1.
3 K. Ono, M. M. Condron and D. B. Teplow, Proc. Natl. Acad. Sci.U. S. A., 2009, 106, 14745.
4 G. M. Shankar, S. M. Li, T. H. Mehta, A. Garcia-Munoz,N. E. Shepardson, I. Smith, F. M. Brett, M. A. Farrell, M. J. Rowan,C. A. Lemere, C. M. Regan, D. M. Walsh, B. L. Sabatini andD. J. Selkoe, Nat. Med., 2008, 14, 837.
5 W. M. Kok, D. B. Scanlon, J. A. Karas, L. A. Miles, D. J. Tew,M. W. Parker, K. J. Barnham and C. A. Hutton, Chem. Commun.,2009, 6228.
6 B. O’Nuallain, D. B. Freir, A. J. Nicoll, E. Risse, N. Ferguson,C. E. Herron, J. Collinge and D. M. Walsh, J. Neurosci., 2010,30, 14411.
7 L. Wu, P. Rosa-Neto, G. Y. Hsiung, A. D. Sadovnick, M. Masellis,S. E. Black, J. Jia and S. Gauthire, Can. J. Neurol. Sci., 2012, 39, 436.
8 J. C. Janssen, J. A. Beck, T. A. Campbell, A. Dickinson, N. C. Fox,R. J. Harvey, H. Houlden, M. N. Rossor and J. Collinge, Neurology,2003, 60, 235.
9 J. Shearer, P. C. Callan, T. Tran and V. A. Szalai, Chem. Commun.,2010, 46, 9137.
10 L. Yu, R. Edalji, J. E. Harlan, T. F. Holzman, A. P. Lopez, B. Labovsky,H. Hillen, S. Barghorn, U. Ebert, P. L. Richardson, L. Miesbauer,L. Solomon, D. Bartley, K. Walter, R. W. Johnson, P. J. Hajduk andE. T. Olejniczak, Biochemistry, 2009, 48, 1870.
11 See ESI†.12 S. Barghorn, V. Nimmrich, A. Striebinger, C. Krantz, P. Keller,
B. Janson, M. Bahr, M. Schmidt, R. S. Bitner, J. Harlan, E. Barlow,U. Ebert and H. J. Hillen, J. Neurochem., 2005, 95, 834.
13 J. Peisach and W. E. Blumberg, Arch. Biochem. Biophys., 1974,165, 691.
14 B. Alies, H. Eury, C. Bijani, L. Rechignat, P. Faller and C. Hureau,Inorg. Chem., 2011, 50, 11192.
15 (a) R. A. Himes, G. Y. Park, A. N. Barry, N. J. Blackburn andK. D. Karlin, J. Am. Chem. Soc., 2007, 129, 5352; (b) R. A. Himes,G. Y. Park, G. S. Siluvai, N. J. Blackburn and K. D. Karlin, Angew.Chem., Int. Ed., 2008, 47, 9084; (c) J. Shearer and V. A. Szalai, J. Am.Chem. Soc., 2008, 130, 17826.
16 L.-S. Kau, D. J. Spira-Solomon, J. E. Penner-Hahn, K. O. Hodgsonand E. I. Solomon, J. Am. Chem. Soc., 1987, 109, 6433.
17 I. W. Hamley, Chem. Rev., 2012, 112, 5147.18 A. Baumketner, S. L. Bernstein, T. Wyttenbach, G. Bitan,
D. B. Tepplow, M. T. Bowers and J.-E. Shea, Protein Sci., 2006,15, 420.
19 P. A. Adlard and A. I. Bush, J. Alzheimer’s Dis., 2006, 10, 145.20 H. A. Feaga, R. C. Maduka, M. N. Foster and V. A. Szalai, Inorg.
Chem., 2011, 50, 1614.
Fig. 3 Snapshots of the Ab(42)H(6)R (top) and wild-type Ab(42) (bottom)hexamers corresponding to lowest-energy structures from the MD simulation.H(13), H(14) and R(6) (or H(6)) have been highlighted.
ChemComm Communication
Publ
ishe
d on
23
Apr
il 20
13. D
ownl
oade
d by
Hei
nric
h H
eine
Uni
vers
ity o
f D
uess
eldo
rf o
n 25
/09/
2013
11:
24:1
4.
View Article Online



![Radical Cation π‐Dimers of Conjugated Oligomers as ... › contents › ... · transport through molecular wires has been pointed out.[43-47] This intimate relationship was deduced](https://static.fdocument.org/doc/165x107/5f0c70957e708231d43568ca/radical-cation-adimers-of-conjugated-oligomers-as-a-contents-a-.jpg)

![Trasporto O2 nel sangue - med.unipg.it Didattico/Fisiologia (Grassi... · Curva di saturazione dell’emoglobina [HbO 2] Hbtotale x 100 Saturazione in O 2 (%) = [HbO2] Hbtotale ...](https://static.fdocument.org/doc/165x107/5c65ba0909d3f28c6e8d3cc9/trasporto-o2-nel-sangue-medunipgit-didatticofisiologia-grassi-curva.jpg)