Endoglin negatively regulates transforming growth factor β1 induced
Cloning and Sequence Analysis of Simian Transforming Growth Factor-β cDNA
Transcript of Cloning and Sequence Analysis of Simian Transforming Growth Factor-β cDNA

DNAVolume 6, Number 3, 1987Mary Ann Liebert, Inc., PublishersPp. 239-244
Cloning and Sequence Analysis ofSimian Transforming Growth Factor-ß cDNA
KENNETH SHARPLES, GREGORY D. PLOWMAN, TIMOTHY M. ROSE,DANIEL R. TWARDZIK, and A.F. PURCHIO
ABSTRACT
We have isolated a cDNA clone coding for the simian TGF-/3 precursor from a cDNA library made from anAfrican green monkey cell line, BSC-40. The deduced amino acid sequence of the mature simian TGF-/3shows 100% homology with that of the human TGF-/3: Strong sequence homology was found between theprecursor regions of the human and simian proteins with only five amino acid changes out of 278. Thesimian (and murine) precursor sequence was found to code for one less amino acid residue than the human.The implications of these results with respect to the isolation of a growth inhibitor from these cells function-ally related to the TGF-ß molecule are discussed.
INTRODUCTION
THE TRANSFORMING GROWTH FACTOR (TGF) family ofgrowth-modulating peptides consists of two structur-
ally and functionally dissimilar molecules, TGF-a andTGF-/3. TGF-a, synthesized and released by retrovirus-transformed rodent cell lines (DeLarco et ai, 1978; Tward-zik et ai, 1982) and some human tumor cell lines (Todaroet ai, 1980), competes with epidermal growth factor (EGF)for binding to (Todaro et ai, 1976) and stimulating tyro-sine-specific phosphorylation of the EGF receptor(Reynolds et ai, 1981). A potent mitogen for cells of mes-
enchymal origin, the mature 50-amino-acid form of TGF-ashares sequence homology with both rodent and humanEGF (Marquardt et ai, 1983) and is cleaved from a
159-amino-acid precursor (Derynck et al, 1984; Lee et ai,1985).
TGF-/3, a disulfide-linked homodimer (nine cysteines/chain), contains two identical 112-amino-acid subunits andutilizes a receptor distinct from either TGF-a or EGF (Fro-lik et ai, 1984; Tucker et ai, 1984a). This potent modula-tor of cell behavior is synthesized by a variety of normaland transformed cells in culture (Roberts et ai, 1981) andhas been purified from various sources including placenta(Frolik et ai, 1983), kidney (Roberts et ai, 1983), urine(Twardzik and Sherwin, 1985), and blood platelets (Childset ai, 1982). TGF-/3 requires either TGF-a or EGF to pro-mote the anchorage-independent growth of normal rat kid-
ney (NRK) fibroblasts whereas this requirement is lessstringent on AKR-2 murine indicator cells (Tucker et ai,1983). In contrast to stimulating cell growth, TGF-/3 fromhuman platelets and a functionally related polypeptide withnearly identical biochemical properties isolated fromAfrican green monkey cells (BSC-1) has also been shown toexhibit growth-inhibitory effects on some cells in culture(Tucker et ai, 1984b). Both the bifunctionality of TGF-/3(inhibition/stimulation) and its apparent ubiquitous distri-bution suggest that it may play a key role in regulating thegrowth and behavior of mammalian cells.
The amino acid sequence deduced from cDNAs encod-ing the TGF-/3 precursor of human (Derynck et ai, 1985)and mouse (Derynck et ai, 1986) origin indicate a high de-gree of homology not only in the area of the mature TGF-/3sequence but also in the amino-terminal precursor region.Since the growth inhibitor isolated by Tucker et ai (1984b)from an African green monkey cell line was inferred to besimilar to TGF-/3, it was of interest to determine if the cellline contained rnRNA for this growth factor. In this study,we report the cDNA cloning of the TGF-/3 precursor froma related cell line (BSC-40, a subline of BSC-1 cells) previ-ously shown to produce high levels of a growth inhibitorfunctionally related to TGF-/3. Exceedingly strong se-
quence homology between the simian precursor and thegene product predicted for both human and mouse TGF-/3are found.
ONCOGEN, 3005 First Avenue, Seattle, WA 98121.
239

240 SHARPIES F:T AL.
MATERIALS AND METHODSGrowth of cells and RNA extraction
BSC-40 cells were grown in Dulbecco's modified Eagle'smedium cntaining 10% fetal calf serum. MCF-7 cells were
grown in the same medium containing 6 U/ml insulin.Poly(A)-containing RNA was isolated from these cells byoligo(dT)-cellulose chromatography exactly as described(Purchio and Fareed, 1979).
cDNA library construction and screeningDouble-stranded cDNA was synthesized from BSC-40
polyadenylated RNA as described in Maniatis et al. (1982)and after treatment with Eco RI methylase was ligated toEco RI linkers. The cDNA was digested with Eco RI andfractionated by chromatography on Sephacryl S-1000.cDNA fractions greater than 750 bp were pooled andligated into fco RI-cut XgtlO (Davis et ai, 1980), packaged(Grosveld et ai, 1981), and plated on E. coliC600rK"mK*hfl. The library was screened by plaque hybrid-ization (Benton and Davis, 1977) to a 32P-labeled oligonu-cleotide probe [5-CACGCAGCAGTTCTTCTCCGTGG-AGCTGAAGCAATA-3'] complementary to codons 6-17of the mature TGF-/3 molecule (Derynck et ai, 1985). Sev-eral cDNA clones were isolated after tertiary screening andsubcloned into pBR322. One clone (pTGF-/3-2) containinga 1600-bp insert was subcloned into the M13mpl8 andM13mpl9 cloning vectors (Yanisch-Perron et ai, 1985) andsequenced on both strands using the dideoxy chain-termi-nation method (Sanger et ai, 1977).
Northern blot analysisPolyadenylated RNA was isolated from MCF-7 cells and
BSC-40 cells as described (Purchio and Fareed, 1979), frac-tionated on a 1% agarose-formaldehyde gel (Lehrach etai, 1977), transferred to a nylon membrane (Hybond,Amersham), and hybridized to 32P-labeled pTGF-0-2probe. Hybridization was carried out at 42°C in 50% form-amide containing 0.9 M NaCl, 50 mM sodium phosphate(pH 7.0), 5 mMEDTA, 0.1% NaDodSO,, 4x Denhardt'ssolution (Maniatis et ai, 1982), 0.4 mg/ml of yeast tRNA,and 0.25 mg/ml of denatured calf thymus DNA. Filterswere washed at 65°C in 0.25 x SSC (Maniatis et ai, 1982),0.2% NaDodSO,,, dried, and exposed to Cronex-4 X-rayfilm (DuPont) with the aid of Lightning Plus intensifierscreens (DuPont).
RESULTS
A cDNA library was constructed in XgtlO using poly(A)-containing RNA from BSC-40 cells as described in thelegend to Fig. 1. The library was screened with a 36-mer de-oxyoligonucleotide complementary to codons 6-17 of themature TGF-/3 molecule (Derynck et ai, 1985). Due to theclose similarity between bovine (Roberts et ai, 1983) andhuman (Derynck et ai, 1985) TGF-/3, we expected that a
probe derived from human sequences would hybridize tomonkey TGF-/3 cDNA clones.
A total of 13 positive clones was identified after threerounds of plaque purification. A 1600-bp clone, pTGF-/3-2,which appeared by restriction enzyme analysis to containthe entire TGF-/3 precursor coding region, was chosen forsequencing. The DNA sequence, along with the deducedamino acid sequence, is shown in Fig. 1. A single openreading frame was found coding for a 390-amino-acid poly-peptide. The mature TGF-/3 polypeptide consists of the car-
boxy-terminal 112 residues. This arrangement is the same
as that described for human (Derynck et ai, 1985) andmouse (Derynck et ai, 1986) TGF-/3. The simian cDNAclone is 98% homologous to the human cDNA clonethroughout the coding region with only 27 mismatches(Fig. 1) and one gap of three bases.
Within the 5'-noncoding region shared by the two clones,the DNA sequences are 91% homologous with three gapswhile the 3'-noncoding regions are 94% homologous withno gaps. Just upstream from the 5' initiating codon is a po-tential stem structure which is also present in the humancDNA clone but not in the mouse clone. An apparent inser-tion within this region in the mouse clone would inhibit for-mation of this structure. [Note that the DNA sequence atthe initiating methionine codon does not conform to theconsensus sequence ACCATGG reported to be optimal foreukaryotic initiating sites (Kozak, 1986).] Like the humanand mouse clones, the simian cDNA clone was not fulllength as no 3' poly(A) track was identified. The G-C-richnature of the 3' end of the TGF-/3 precursor mRNA mayhave resulted in a secondary structure which preventedDNA synthesis through this region. The 3' noncoding re-
gion exhibits a remarkable repetition of the sequence(purine) CCCC following the termination codon. This se-
quence occurs nine times in the human sequence with an
additional repeat containing one base difference. Both thesimian and murine clones contain eight repetitions with an
additional two containing a single base difference.Northern blot analysis using the TGF-/3-2 probe showed
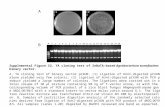
hybridization to a major 2.5-kb polyadenylated RNA spe-cies from BSC-40 cells and the human mammary carci-noma cell line MCF-7 (Fig. 2). This is the same size as themajor transcript found in human (Derynck et ai;, 1985)and mouse (Derynck et ai, 1986) cell lines. Minor bands at4 kb and 1.45 kb can also be seen in lane 2 of Fig. 2.
Figure 1 also shows the amino acid homology betweenthe human and monkey TGF-/3 precursor proteins. Onlyfive amino acid changes occur, all of which are foundwithin the amino-terminal precursor region: There is com-
plete homology between the simian and human matureTGF-/3 molecule at the amino acid level. In comparison,84% homology (at the amino acid level) was found to existbetween the monkey and mouse precursor TGF-/3 mole-cules: one amino acid change, a serine (mouse) to an ala-nine (monkey) was found in the mature TGF-/3 codingregion. The human gene codes for an extra amino acid(arginine, position 158) in the precursor which is present inneither the simian nor murine clone. Presumably thischange is due to an insertion within the human gene thatwas sequenced (Derynck et ai, 1985). The close identity be-tween the precursor region of the monkey, mouse, and hu-man TGF-0 protein suggests that this part of the moleculemay also have an important biological function.

SIMIAN TGF-(3 cDNA 241
Simi anHuman
-261 RGGGGRTCTGTGGCRGGTCGGRGR-RRGRTC-CGTCTCCTGGTRCCRGRTCTCGCCCRTCTRGGTT -198CTCC.
.
C.. .
CCR..
.Pi..
CCCT.
TTC.. .
.C.RCC.RC..
T.G.
Simian RTTTCC6TGGGRTRCTGRGRCRCCCCCGGTCCRRGCCTCCCCTCCRCCRCTGCGCCCTTCTCCCTGRGGR-CCTCRRCTTTCCCTCGRGGCCCTCCTRC -lOOHuman
Simian CTTTTCCCGGGGGRCCCCCRGCCCCTGCRGGGGCGGGGCCTCCCCRCCRRRCTRGCCCTGTTCGCGCTCTCGGCRGTGCCGGGGGGCGCCGCCTCCCCCHuman .G.R.C..C.
^a^^^Tu" ThrGit,SarMat Pr-O Pro Sar- Gly Lau Rr-g Lau Lau Pro Lau Lau Lau fToT^»Tj^lTT^T^5^lTT
Simian RTB CC6 CCC TCC GGG CTG CGG CTG CTG CCG CTG CTG CTR CCG CTG CTG TQG CTR CTG GTG CTG RCG CCT RGC CGGHuffl«n.G.
SimianHuman
35 45 R1 a
Pro Rla Rla Gly Lau Sar- Thr- Cym Lu« Thr lia R«p Mat Glu Lau Val Ly« Rrg Lys Rr-g lia Glu Thr- lia Rr-gCCG GCC GCft GGR CTR TCC RCC TGC RR6 RCT RTC GRC RTG GRG CTG GTG RRG CGG RRG CGC RTC GRG RCC RTC CGC
SimianHuman
60 70Gly Gin lia Lau Sar- Ly» Lau Rr-g Lau Rla Sar- Pr-a Pro Sar- Gin Gly Glu Val Pro Pr-o Gly Pr-o Lau Pr-o GluGGC CRG RTC CTG TCC RRG CTG CGG CTC GCC RGC CCC CCG RGC CRG GGG GRG GTG CCG CCC GGC CCG CTG CCC GRG
SimianHuman
85Rr-gftla Val Lau Ria Lau Tyr- Ran Sar- Thr- Rr-g R»p Rr-g Val Ria Gly Glu Sar- Ria Glu Pr-o Glu Pr-o Glu Pr-o Glu
GCC GTG CTC GCC CTG TRC RRC RGC RCC CGC GRC CGG GTG GCC GGG GRG RGT GCG GRG CCG GRG CCC GRR CCG GRG
SimianHuman
fll* Rap Tyr- Tyr- Ria Ly» Glu Val Thr Mat Val Glu Thn120I la R»p Ly» Pha Ly»
GCC GRC TRC TRC GCC RRG GRG GTC RCC CGC GTG CTR RTG GTG GRR RCC CRC RRC GRR RTC TRT GRC RRG TTC RRG
135 145Gin Sar- Thr Hi a San II» Tyr Mat Pha Pha R«n Thr Sar- Glu Lau Rr-g Glu Rla Val Pr-o Glu Pro Val Lau Lau
Simian CRG RGC RCR CRC RGC RTR TRT RTG TTC TTC RRC RCR TCR GRG CTC CGR GRR GCR GTR CCT GRR CCT GTG TTG CTC 450
Human .T.G.C.
Rng 160 170Sar Rng Rla Glu Lau Rng Lau Lau
-
Rrg Lau Ly» Lau Ly« Val Glu Gin Hi» Val Glu Lau Tyr Gin Ly» TyrSimian TCC CGG GCR GRG CTG CGT CTG CTG
-
RGG CTC RRG TTR RRR GTG GRG CRG CRT GTG GRG CTG TRC CRG RRR TRC 522
Human .RGG.C.185 R»p 195
Sar R»n R»n Sar Tnp Rrg Tyr Lau Sar R^r, Rrg Lau Lau Ria Pro Sar Ran Sar Pro Glu Trp Lau Sar Pha R»pSimian RGC RRC RRT TCC TGG CGR TRC CTC RGC RRC CGG CTG CTG GCG CCC RGC RRC TCG CCG GRG TGG TTG TCT TTT GRT 597
Human.R.G.R.R.
210 220
Val Thr Gly Val Val Rrg Gin Trp Lau Sar Rrg Gly Gly Glu lia Glu Gly Pha Rrg Lau Sar Ria Hi« Cy» Sar
Simian GTC RCC GGR GTT GTG CGG CRG TGG TTG RGC CGC GGR GGG GRR RTT GRG GGC TTT CGC CTT RGC GCC CRC TGC TCC 672
Human.T.
Rrg 235 245
Cy» R»p Sar Ly» R»p R^r-, Thr Lau Gin Val R»p lia R«n Gly Ph» Thr Thr Gly Rrg Rrg Gly R»p Lau Ria Thr
Simian TGT GRC RGC RRR GRT RRC RCR CTG CRR GTG GRC RTC RRC GGG TTC RCT RCC GGC CGC CGR GGT GRC CTG GCC RCR 747
Human .GG.C260 270
11a Hi» Gly Mat Rmr\ Rng Pno Pha Lau Lau Lau Mat Ria Thr Pro Lau Glu Rrg Ria Gin Hi» Lau Gin Sar Sar
Simian RTT CRT GGC RTG RRC CGG CCT TTC CTG CTT CTC RTG GCC RCC CCR CTG GRG RGG GCC CRR CRT CTG CRR RGC TCC 32 3
Human .G.G.
S1m1 anHuman
Rrg Hi» Rrg RngCGG CRC CGC CGR
rn"a^Laûfi»^^rT^»^^yr^Cy»^Prîa^Sar^5Tr^Tr?r™T^^^LGCC CTG GRC RCC RRC TRC TGC TTC RGC TCC RCG GRG RRG RRC TGC TGC GTG CGG CRG CTG TRT
.T.C"af-JT
iUL. LaRÎ^PhTcL^a^îïp^nna^Çn^^L^ï^RapTîaT^^^y^Tn^^Lyï^Tn^^^^r^H^ Ria R»n Pha Cy» Lau GlySimian RTT GRC TTC CGC RRG GRC CTC GGC TGG RRG TGG RTC CRC GRG CCC RRG GGC TRC CRT GCC RRC TTC TGC CTG GGG
Human.C
-if?ä-Simian CCC TGT CCC TRC RTT TGG RGC CTG GRC RCG CRG TAC RGC RRG GTC CTG GCC CTG TRC RRC CRG CRT RRC CCG GGC
Human .C. .. •
.
ST San SI. Al. Pr-o Su. gy« U.l Pno Bin IfÜ Lau 51u Pno L. 'Tun Tyr- Olí Ely Rr-g Ly.Simian GCC TCG GCG GCG CCG TGC TGC GTG CCG CRG GCG CTG GRG CCR CTG CCC RTC GTG TRC TRC GTG GGC CGC RRG CCC
Human.G.
L^T^aTT?T^T?r!^Tu^ar^ïnTïTT^î^JaTTrT^ÇrnTy^^^^Ty,^^r?Simian RRG GTG GRG CRG CTG TCC RRC RTG RTC GTG CGC TCC TGC RRR TGC RGC
Human.
. . . - . . .
G.
SimljHumar
TGR GGCCCCGCCCCGCCCCGCCCCRCCCCGGCRG.T.G.
GCCCGGCCCCGCCCCRCCCCRCCCCCGCTGTCTTGlfcCTTGGGGGCTGTRTTTRRGGRCRCCCGTGCCCCRflGCCCRCCTGGGGCCCCRTTRRRGR.R.... G
....
G.C. . . .
i
. .
R.
FIG. 1. Nucleotide sequence of simian TGF-0 cDNA and deduced amino acid sequence. The 1600-bp insert of pTGF-/3-2 was subcloned into the M13mpl8 and M13mpl9 cloning vectors (Yanisch-Perron et ai, 1985) and sequenced on bothstrands using the dideoxy chain-termination method (Sanger et ai, 1977). The simian DNA sequence is shown and the de-duced amino acid sequence is presented directly above. The human and simian DNA sequences are identical (dots) exceptwhere indicated. Amino acid differences between the human and simian proteins are shown in the top line. The matureTGF-0 sequence is boxed and the signal peptide is overlined. A palindrome in the 5' untranslated region is also overlined.

242 SHARPLES ET AL.
DISCUSSION
28S
' '' "'™:":í*;.-:
1 2
FIG. 2. Northern blot analysis of RNA from a human(MCF-7) and monkey (BSC-40) cell line using a monkeyTGF-0 cDNA probe. Polyadenylated RNA was isolatedfrom MCF-7 cells and BSC-40 cells as described (Purchioand Fareed, 1979), fractionated on a 1% agarose-formalde-hyde gel (Lehrach et ai, 1977), transferred to a nylon mem-brane (Hybond, Amersham), and hybridized to 32P-labeledpTGF-/3-2 probe. Lane 1, MCF-7 RNA (5 fig); lane 2,BSC-40 RNA (5 fig). 28S and 18S indicate the positions ofmigration of 28S and 18S ribosomal RNAs.
The carboxyl terminus of the precursor contains the 112-amino-acid mature form of simian TGF-/3 and is identifiedby its homology to the corresponding human gene product.This cysteine-rich (nine residues) region of the precursorrepresents the secreted form of TGF-/3 and does not con-
tain any putative glycosylation sites, whereas three poten-tial N-glycosylation sites (Asn-82, -136, and -177) can beidentified in other regions of the precursor. It is known thatthe TGF-/3 homodimer, both of human and rodent origin,requires intact disulfide bonds to maintain activity (Mes-sague, 1984). The amino terminus of mature human TGF-/3as established by primary sequence analysis (Derynck et ai,1985) is preceded by an Arg-Arg dipeptide. This similarcleavage site is also present in an identical position in thesimian homolog. Cleavage either during or after the trans-lational process concomitant with, either or both, intra-and interchain (dimerization) disulfide bond formationwould result in the genesis of the active homodimer. In thisregard, the TGF-/3 precursor contains a leucine-rich regionof 16 hydrophobic amino acids (positions 8-21, Fig. 1)which could function as a signal peptide and play a role inthe secretion/processing cascade.
The remarkable conservation in the sequence of TGF-/3among simian, rodent, and human species suggests thatstrong evolutionary pressure was required, by necessity, toconserve important functionality. This applies not only tothe mature form of TGF-/3, which has an important bifunc-tional role as a growth regulator, but also to the polypep-tide region of the precursor upstream from the matureTGF-/3 sequence which may also exhibit coordinate growthmodulatory activities.
The major TGF-/3 mRNA species in BSC-40 cells is 2.5kb, in agreement with results obtained for murine and hu-man TGF-/3-specific messages. The minor bands seen at 4kb and 1.45 kb may represent either aberrantly processedtranscripts or may code for a TGF-ß-like molecule (CIF-B)recently described (Seyedin et ai, 1987) which contains ex-
tensive homology to TGF-/3.The deduced polypeptide sequence of the simian, hu-
man, and mouse clones contains a nearly identical contigu-ous stretch of 16 hydrophobic residues in positions 8-21which may constitute an amino-terminal signal peptide. Inaddition, an Arg-Arg dipeptide immediately precedes theamino terminus of mature TGF-/3, suggesting similarproteolytic cleavage sites. The simian TGF-/3 precursor alsocontains three putative N-glycosylation sites not found inthe mature molecule. Thus, not only has the primary struc-ture of the mature and precursor polypeptide of TGF-/3been conserved among species, but distinct posttransla-tional modification sites as well.
BSC-40 cells synthesize and release high levels of TGF-/3relative to other cell lines we have tested. Media condi-tioned by BSC cells contain an activity which in the pres-ence of nanogram levels of EGF and TGF-a stimulates theanchorage-independent growth of NRK cells and exhibitsbiochemical characteristics identical to TGF-/3 purifiedfrom human platelets (Frolik et at, 1983; Roberts et ai,1983). In addition, Holley et ai have described the isola-tion of a growth inhibitor from BSC-1 cells functionallysimilar to TGF-/3 which competes with platelet-derivedTGF-/3 for binding to TGF-/3 membrane receptors (Tuckeret ai, 1984b). The large amount of TGF-/3-specific mRNAsynthesized by BSC-40 cells, a cell line derived from BSC-1cells, suggests that the bifunctional growth modulator iden-tified by Holley et ai in BSC-1 conditioned media is indeedTGF-/3. This is also supported by recent data which con-
firm Holley's observations that TGF-/3 from other sources
also inhibits the growth of some tumor cells in culture(Roberts et ai, 1985). However, formal proof that theBSC-1-derived inhibitor is indeed the product of the genewe describe in this report will require sequence analysis ofthe TGF-(3-like activity released by BSC-1 cells in culture.Experiments to express active TGF-/3 are in progress. Com-parison of the activities of the purified expressed proteinand the TGF-/3-like activity released by BSC-1 cells shouldalso aid in establishing whether the two molecules are func-tionally identical.

SIMIAN TGF-/3 CDNA 243
ACKNOWLEDGMENT
We wish to thank Nancy Olfs and Deborah Stephens fortheir assistance in the preparation of this manuscript.
REFERENCES
BENTON, W.D., and DAVIS, R.W. (1977). Screening lambda gtrecombinant clones by hybridization to single plaques in situ.Science 196, 180-182.
CHILDS, C.B., PROPER, J.A., TUCKER, R.F., and MOSES,H.L. (1982). Serum contains a platelet-derived transforminggrowth factor. Proc. Nati. Acad. Sei. USA 79, 5312-5316.
DAVIS, R.W., BOTSTEIN, D., and ROTH, J.R. (1980). In AManual for Genetic Engineering: Advanced Bacterial Genetics.Cold Spring Harbor Laboratory, Cold Spring Harbor, NewYork.
DeLARCO, J.E., PRESTON, Y.A., and TODARO, G.J. (1978).Growth factors from murine sarcoma virus-transformed cells.Proc. Nati. Acad. Sei. USA 75, 4001-4005.
DERYNCK, R., JARRETT, J.A., CHEN, E.Y., EATON, D.H.,BELL, J.R., ASSOIAN, R.K., ROBERTS, A.B., SPORN,M.B., and GOEDDEL, D.V. (1985). Human transforminggrowth factor-(3 complementary DNA sequence and expressionin normal and transformed cells. Nature 316, 701-705.
DERYNCK, R., JARRET, J.A., CHEN, E.Y., and GOEDDEL,D.V. (1986). The murine transforming growth factor-ß precur-sor. J. Biol. Chem. 261, 4377-4379.
DERYNCK, R., ROBERTS, A.B., WINKLER, M.E., CHEN,E.Y., and GOEDDEL, D.V. (1984). Human transforminggrowth factor-a: Precursor structure and expression in E. coli.Cell 38, 287-297.
FROL1K, CA., DART, L.L., MEYERS, CA., SMITH, D.M.,and SPORN, M.B. (1983). Purification and initial characteriza-tion of a type ß transforming growth factor from human pla-centa. Proc. Nati. Acad. Sei. USA 80, 3676-3680.
FROLIK, CA., WAKEFIELD, L.M., SMITH, D.M., andSPORN, M.B. (1984). Characterization of a membrane recep-tor for transforming growth factor type ß in normal rat kidneycells. J. Biol. Chem. 260, 10995-11000.
GROSVELD, F.G., DAHL, H.M., DeBOER, E., and FLA-VELL, R.A. (1981). Isolation of beta-globin-related genes froma human cosmid library. Gene 13, 227-237.
KOZAK, M. (1986). Point mutations define a sequence flankingthe AUG initiator codon that modulates translation by eukary-otic ribosomes. Cell 44, 283-292.
LEE, D.C, ROSE, T.M., WEBB, N.R., and TODARO, G.J.(1985). Cloning and sequence analysis of a cDNA for rat trans-
forming growth factor-a. Nature 313, 489-491.LEHRACH, H., DIAMOND, D., WOZNEY, J.M., and
BOEDTKER, H. (1977). RNA molecular weight determinationby gel electrophoresis under denaturing conditions: A critical re-
examination. Biochemistry 16, 4743-4751.MANIATIS, T., FRITSCH, E.F., and SAMBROOK, J. (1982).
In Molecular Cloning: A Laboratory Manual. Cold Spring Har-bor Laboratory, Cold Spring Harbor, New York. pp. 371-372.
MARQUARDT, H., HUNKAPILLER, M.W., HOOD, L.E.,TWARDZIK, D.R., DeLARCO, J.E., STEPHENSON, J.R.,
and TODARO, G.J. (1983). Transforming growth factors pro-duced by retrovirus-transformed rodent fibroblasts and humanmelanoma cells: Amino acid sequence homology with epidermalgrowth factor. Proc. Nati. Acad. Sei. USA 80, 4684-4688.
MASSAGUE, J. (1984). Type ß transforming growth factor fromfeline sarcoma virus-transformed rat cells. J. Biol. Chem. 259,9756-9761.
PURCHIO, A.F., and FAREED, G.C. (1979). Transformationof human embryonic kidney cells by human papovavirus BK. J.Virol. 29, 763-769.
REYNOLDS, S.F., TODARO, G.J., FRYLING, G.C, andSTEPHENSON, J.R. (1981). Human transforming growth fac-tors induce tyrosine phosphorylation of EGF receptors. Nature292, 259-262.
ROBERTS, A.B., ANZANO, M.A., LAMB, L.C., SMITH, J.M.and SPORN, M.B. (1981). New class of transforming growthfactors potentiated by epidermal growth factor: Isolation fromnon-neoplastic tissues. Proc. Nati. Acad. Sei. USA 78, 5339-5343.
ROBERTS, A.B., ANZANO, M.A., MEYERS, CA., WIDE-MAN, T., BLACHER, R., PAN, Y.E., STEIN, S., LEHR-MAN, S.R., SMITH, T.M., LAMB, L.C, and SPORN, M.B.(1983). Purification and properties of a type ß transforminggrowth factor from bovine kidney. Biochemistry 22, 5692-5698.
ROBERTS, A.B., ANZANO, M.A., WAKEFIELD, L.M.,ROCHE, N.S., STERN, D.E., and SPORN, M.B. (1985). Typeß transforming growth factor: A bifunctional regulator of cellgrowth. Proc. Nati. Acad. Sei. USA 82, 119-123.
SANGER, F., NICKLEN, S., and COULSON, A.R. (1977). DNAsequencing with chain-terminating inhibitors. Proc. Nati. Acad.Sei. USA 74, 5463-5467.
SEYEDIN, S.M., SEGARINI, P.R., ROSEN, D.M., THOMP-SON, A.Y., BENTZ, H., and GRAYCAR, J. (1987). Cartilageinducing factor-B is a unique protein structurally and function-ally related to transforming growth factor-0. J. Biol. Chem. (inpress).
TODARO, G.J., DeLARCO, J.E., and COHEN, S. (1976).Transformation by murine and feline sarcoma viruses specif-ically blocks binding of epidermal growth factor (EGF) to cells.Nature 264, 26-29.
TODARO, G.J., FRYLING, CM., and DeLARCO, J.E. (1980).Transforming growth factors produced by certain human tumorcells: Polypeptides that interact with epidermal growth factorreceptor. Proc. Nati. Acad. Sei. USA 77, 5258-5262.
TUCKER, R.F., BRANUM, F.L., SHIPLEY, G.D., RYAN,R.J., and MOSES, H.L. (1984a). Specific binding to culturedcells of l2T-labeled type ß transforming growth factor from hu-man platelets. Proc. Nati. Acad. Sei. USA 81, 6757-6761.
TUCKER, R.F., SHIPLEY, CD., MOSES, H.L., and HOL-LEY, R.W. (1984b). Growth inhibitor from BSC-1 cells closelyrelated to platelet type ß transforming growth factor. Science226, 705-707.
TUCKER, R.F., VOLKENANT, M.E., BRANUM, E.L., andMOSES, H.L. (1983). Comparison of intra- and extracellulartransforming growth factors from nontransformed and chemi-cally transformed mouse embryo cells. Cancer Res. 43, 1581-1586.
TWARDZIK, D.R., and SHERW1N, S. (1985). Transforminggrowth factor activity (TGF) in human urine: Synergism be-tween 0-TGF and urogastrone. J. Cell. Biochem. 28, 289-297.
TWARDZIK, D.R., TODARO, G.J., MARQUARDT, H.,REYNOLDS, F.H., and STEPHENSON, J.R. (1982). Trans-formation induced by Abelson murine leukemia virus involves

244 SHARPIES ET AL.
production of a polypeptide growth factor. Science 216, 894-897.
YANISCH-PERRON, C, VIEIRA, J., and MESSING, J. (1985).Improved MI3 phage cloning vectors and host strains: Nucleo-tide sequences of the M13mpl8 and pUC19 vectors. Gene 33,103-119.
Address reprint requests to:Dr. A.F. Purchio
ONCOGEN3005 First A venue
Seattle, WA 98121
Received for publication January 9, 1987.