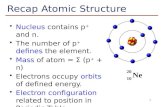
Atomic Theory & Structure - Weebly
Transcript of Atomic Theory & Structure - Weebly
c = λνE = hν
c = speed of light (2.998 x 108 m/s)
λ = wavelength (m) ν = frequency (Hz) h = Planck’s constant
(6.626 x 10-34 J·s)
Photons of light shining on a metal causes electrons to be emitted from the surface.
What energy does the photon need to cause this to happen?
Only orbits, corresponding to these energies, exist for the electron in a hydrogen atom.
Electrons in these allowed energy state do not radiate energy, and as a result, does not spiral in to the radius.
As an electron changes energy states, it emits or absorbs energy as a photon with an energy equal to the difference in the two energy states.
Bohr just assumed that electrons would not spiral into the nucleus.
He didn’t explain WHY this doesn’t happen!
Bohr’s model says that all electrons in the same principal energy level have the same energy.
Photoelectron spectroscopy (PES) proves that this isn’t true!
It is impossible for us to know the exact momentum (energy) and exact location (orbital) of an electron at the same time!
This opened the door for the Quantum Mechanical Model.
Describes the probability of finding an electron with a given energy in a certain region of space in an atom.
Principal QN (n) = 1, 2, 3, …
Higher n Larger orbital (further from nucleus) with higher energy
Angular momentum QN (l) = 0, 1, 2, …, (n-1)
Shape of orbital (0 = s, 1 = p, 2 = d, 3 = f)
Magnetic QN (ml) = -l, …, -1, 0, 1, …, l
Orientation of orbital around nucleus
Spin QN (ms) = +1/2, -1/2
Spin of electron
No two electrons can have the same set of 4 quantum numbers
Maximum of 2 electrons in the same orbital
Electrons in the same orbital must have opposite spins
For degenerate orbitals, the lowest energy exists when there are the maximum number of electrons with the same spin.