ANALYTICAL PERFORMANCE CHARACTERISTICS OF A … · analytical performance characteristics of a...
Transcript of ANALYTICAL PERFORMANCE CHARACTERISTICS OF A … · analytical performance characteristics of a...

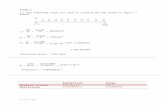
BACE-1 immunoassay principle
ANALYTICAL PERFORMANCE CHARACTERISTICS OF A NOVEL IMMUNOASSAY FOR QUANTIFICATION OF BACE-1 IN HUMAN CEREBROSPINAL FLUID
Emeric CHASSAING1, Mélanie BODNAR-WACHTEL1, Tanja SCHUBERT1, Hugo VANDERSTICHELE2, Erik STOOPS2, and Philippe VERGNAUD1
1Bioclinica LAB, Lyon, France 2ADx NeuroSciences, Gent, Belgium
Accumulation of amyloid-β (Aβ) into plaques in the brain is one of the hallmarks of
Alzheimer’s Disease (AD). This neurotoxic peptide is produced by the consecutive
cleavage of amyloid precursor protein (APP) by β-site APP Cleaving Enzyme 1 (BACE1)
and γ-secretase. Due to its key role in amyloid plaque formation, BACE-1 is considered
as a major therapeutic target for reducing the levels of amyloid in AD brain. The BACE1
ELISA quantifies specifically BACE-1 protein. In contrast to BACE-1 enzyme activity
assays, it does not suffer from a BACE-2 interference.
This study documents the analytical performance characteristics of a novel ELISA for
BACE-1 protein in human cerebrospinal fluid.
I N T RO D U C T I O N
M E T H O D
BACE-1 protein (Size: 55.8 kDa) is quantified in human CSF with a (manual) sandwichELISA, developed by ADx NeuroSciences (Gent, Belgium), and commercialized byEUROIMMUN (Lübeck, Germany).
The Bioclinica LAB analytical validation method is designed to ensure the accuracy andreliability of the results. The validation process, the criteria applied, and theparameters chosen are optimized based on current guidelines, field experience andperformance requirements. The Laboratory is CAP accredited (College of AmericanPathologists).
The CSF samples for the study are provided by 2 different centers (Cerba Specimen &AMS bio). All CSF samples were stored at -70°C. The sample concentrationdetermination is based upon duplicate analysis. LoBind tubes from Eppendorf wereused (ref. 0030108094) for every step in the handling of CSF.
The BACE-1 colorimetric ELISA shows acceptable analytical performance characteristics, such
as good repeatability and reproducibility of the results. Samples can be diluted until 1:8
without showing any matrix interference. The samples tested fall within the dynamic range of
the assay. BACE-1 remains stable after 4 freeze/thaw cycles and 24 hours at room temperature
or +4°C.
This novel assay meets all internal acceptance criteria for CSF samples testing and represents
an additional tool for patient management in research clinical trials.
TA K E H O M E M ES SAG E
PA R A L L E L I S M A N D S P I K E R E C O V E R Y
A C C E P T A B L E R E C O V E R Y R A N G E : 8 0 - 1 2 0 %
Alzheimer's Association International Conference | July 16 – 20 , 2017 | London, England
CSF Sample (pg/mL)
Calibrator(pg/mL)
Expectedvalues
(pg/mL)
Spikedsamples(pg/mL)
Recovery%
R1 923
10752 3380 3 587 106%
7291 2515 2 682 107%
1076 961 900 94%
272 760 781 103%
R2 2365
10752 4461 4 867 109%
7291 2731 2 699 99%
1076 2042 1 973 97%
272 1841 1 768 96%
Mean 101%
Six samples (FS1 – FS6) with different concentrations werediluted subsequently until 1:16 in sample buffer. The recoverypercentages are then calculated as followed:
Recovery % =(𝑚𝑒𝑎𝑠𝑢𝑟𝑒𝑑 𝑣𝑎𝑙𝑢𝑒 𝑋 𝑑𝑖𝑙𝑢𝑡𝑖𝑜𝑛 𝑓𝑎𝑐𝑡𝑜𝑟)
𝐸𝑥𝑝𝑒𝑐𝑡𝑒𝑑 𝑣𝑎𝑙𝑢𝑒x 100
Two CSF samples with different concentrations were spikedwith 4 different concentrations of calibrators in a 75:25 ratio(sample:calibrator). The assay value is then compared to theexpected value.
QC1 QC2 QC3 QC4 QC5
Target (pg/mL) 300 480 1500 2400 4500
Inter CV% 8.0 10.9 4.6 7.1 4.6
Intra CV% 4.7 4.2 4.8 4.5 2.1
Five Quality Control (QC) samples covering the dynamic range of theassay (272 – 10752 pg/mL) were used to determine the Intra-Assayvariation. QCs were repeated 16 times. For the Inter-Assay variation,QCs were measured in 5 different assays on 5 different days.
272
1076
2148
3830
5561
7291
10752
0.0
0.2
0.4
0.6
0.8
1.0
1.2
1.4
1.6
1.8
2.0
1 10 100 1000 10000
Op
tica
l Den
sity
CSF-BACE-1 (pg/mL) concentration range of tested CSF
Limit of Detection29.2 pg/mL
Lower Limit of Quantitation
272 pg/mL
Upper Limit ofQuantitation10752 pg/mL
272 pg/mL (first Calibrator)LLOQ was determined with three CSFsamples with low values and was measuredat least 13 times within the same run. Thelowest mean concentration with a CV%between 15 and 20% should be consideredas the lower limit of quantitation.
29.2 pg/mLLOD were determined by adding two SD(standard deviation) to the mean opticaldensity (OD) of 24 replicates of the samplebuffer. The concentration is then back-calculated with the equation of the curve.
S A M P L E S TA B I L I T Y
M A X I M U M T O L E R A T E D F R O M R E F E R E N C E V A L U E : ± 1 5 %
Six CSF samples with different concentrations were thawed and placed 3, 6 and 24 hours at ambient temperature(+22°C ± 2°C) or in a fridge (+4°C ± 2°C). For each time point, the levels of BACE-1 were determined the same day onthe same run, and the results were compared to the reference value (T0).
Six CSF samples were consecutively thawed and frozen 4 times. No effect on 4 freeze/thaw cycles was detected onBACE-1 concentrations. Percentages of variation, when compared to T0, were all below 10%.
-40
-30
-20
-10
0
10
20
30
40
0 3 6 9 12 15 18 21 24
Pe
rce
nta
ge o
f va
riat
ion
fro
m T
0
time (hours)
A-1 A-2 B-1 B-2 C-1 C-2
-40
-30
-20
-10
0
10
20
30
40
0 3 6 9 12 15 18 21 24
Pe
rce
nta
ge o
f va
riat
ion
fro
m T
0
time (hours)
A-1 B-1 B-2 C-1 C-2 D-1
BACE-1 concentrations were back-calculated following a seven points calibration curve (4-PL curve fitting).
W O R K I N G R A N G E & L I M I T S
L L O Q ≥ F I R S T C A L I B R A T O R | C V % f r o m 1 5 t o 2 0 %
P A R A L L E L I S M
S P I K E R E C O V E R Y
I N T R A - A S S A Y V A R I A T I O N S
I N T E R - A S S A Y V A R I A T I O N S
L O W E R L I M I T O F Q U A N T I T A T I O N ( L L O Q )
L I M I T O F D E T E C T I O N ( L O D )
S T A B I L I T Y A T R O O M T E M P E R A T U R E S T A B I L I T Y A T + 4 ° C
F r e e z e / T h a w c y c l e s e f f e c t s
C A L I B R A T I O N C U R V E
P R E C I S I O N A N D A C C U R A C Y
M A X I M U M C V % T H R E S H O L D < 1 5 %
Simultaneous incubation ofsample (15µL undiluted)with biotinylated detectorantibody (100µL) onantibody-coated plates.
Addition of streptavidinperoxidase conjugate (100µL).
Addition of the peroxidasesubstrate.
Stop of reaction by addition ofsulfuric acid followed byreading the absorbance at theappropriate wavelength.
λSubstrate
1 2 3 4
3 h
ou
rsa
t R
T &
Wa
shin
gst
ep
s
30
min
ute
s a
t R
T i
n t
he
da
rk
30
min
at
RT
& W
ash
ing
ste
ps
0
2000
4000
6000
0 4 8 12 16
Co
nce
ntr
atio
n (
pg/
mL)
Dilution factor (1/x)
Sample (%CV)FS1 (8.3) FS2 (10.2) FS3 (10.3)
FS4 (9.5) FS5 (10.6) FS6 (6.5)
-40%
-30%
-20%
-10%
0%
10%
20%
30%
40%
0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17
Pe
rce
nta
ge o
f va
riat
ion
fro
m t
he
me
an
within run replicates
QC1 QC2 QC3 QC4 QC5
-40%
-30%
-20%
-10%
0%
10%
20%
30%
40%
0 1 2 3 4 5 6P
erc
en
tage
of
vari
atio
n f
rom
th
e m
ean
between run replicates
QC1 QC2 QC3 QC4 QC5