3a. Lattice Dynamics - Unigebracco/serpchem/3a_Lattice_vibrations.pdf · • Our wavelike solutions...
Transcript of 3a. Lattice Dynamics - Unigebracco/serpchem/3a_Lattice_vibrations.pdf · • Our wavelike solutions...
3a. Lattice Dynamics3a. Lattice Dynamics
� Introduction
� Lattice Vibrations of 1D crystals
� Monoatomic chain
� Diatomic chain
� Periodic boundary conditions
� Lattice Vibrations of 3D crystals
G. Bracco-Material Science SERP CHEM 1
Lattice Dynamics
G. Bracco-Material Science SERP CHEM 2
In previous lectures we have assumed that the atoms were at rest at their
equilibrium position. This can not be entirely correct
→ Atoms vibrate about their equilibrium position even at absolute zero!
The energy they possess at T=0 is known as zero point energy.
The amplitude of the motion increases as the atoms gain more thermal energy
at higher temperatures.
In this chapter we discuss the nature of atomic motions, referred to as lattice
vibrations.
In crystal dynamics we will use the harmonic approximation → amplitude of
the lattice vibration is small.
At higher temperature some anharmonic effects occur.
Lattice Dynamics
• The atomic position is a lattice position only in average
• We exclude diffusion events: the atom vibrates always around the samelattice point
• Classical mechanics will be initially employed to describe the atomicmotion
• Motion of a system of atoms connected by springs
G. Bracco-Material Science SERP CHEM 3
Lattice vibrations of 1D crystal
Chain of identical atoms
( )2 2
2( ) ( ) ...........
2r a
r a d VV r V a
dr =
− = + +
rR
V(R)
0 r0
Repulsive
Attractivemin
In equilibrium position first derivative is zero. This equation looks like as the potential energy associated of a spring with a spring constant :
G. Bracco-Material Science SERP CHEM 4
Atoms interact with a potential V(r), for small vibrational amplitude it can
be expanded in Taylor’s series around the equilibrium position r=a.
Harmonic approximation: retain terms up to the quadratic one.
� � ������ �
���
Force= ���� �
Monoatomic Chain
• The simplest crystal is the one dimensional chain of identical atoms.
• Chain consists of a very large number of identical atoms with identical masses.
• Atoms are separated by a distance “a”, the lattice periodicity.
• Atoms move only in a direction parallel to the chain.
• As a further approximation: only nearest neighbours interact (short-range forces).
a a a a a a
Un-2 Un-1 Un Un+1 Un+2
G. Bracco-Material Science SERP CHEM 5
If one expands the energy near the equilibrium point for the nth atom and use elastic approximation, Newton’s equation becomes
The force on the nth atom:• Force to the right F
r= �(���� − ��)
• Force to the leftFl= �(�� − ����)
• Total force = Fr- F
l
��� = �(���� − 2�� + ����)
a a
Un-1 Un Un+1
Eqn’s of motion of all atoms are of this form, only the value of
‘n’ varies
G. Bracco-Material Science SERP CHEM 6
Monoatomic Chain
• All atoms oscillate with a same amplitude A and frequency ω. Then we can try
a solution
( ).
0expnn n
duu i A i kx t
dtω ω = = − −
( )0expn nu A i kx tω = −
( ) ( )2.. 2 2 0
2expn
n n
d uu i A i kx t
dtω ω = = −
..2
n nu uω= −
nax n =0nn unax +=Undisplaced
position
Displaced position
G. Bracco-Material Science SERP CHEM 7
Monoatomic Chain
Cancel Common terms ���(����)
G. Bracco-Material Science SERP CHEM 8
m��� = α(����-2��+����)
Monoatomic Chain
mω2���(�������)
= α(���(���
� ����)-2���(��
�
����)+��
�(���������)
0� = na, 0��� = (n + 1)a, 0�� = (n − 1)a
-mω2 =α(����-2+�����)
With some algebra
ω(k)=α
sin(
��
�) → dispersion relation ωωωω(k)
Maximum value ωM=α
e e 2cosika ika ka−+ =
( ) 21 cos 2 sin2
xx
− =
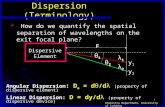
• Dispersion relation: ω versus k relation
G. Bracco-Material Science SERP CHEM 9
ω
k
π/a-π/a
(redundant)
Monoatomic Chain
-�
�� λ �
�
�
λ � ��
�λ �
�
�
• The waves with wave numbers k and k+2π/a describe the
same atomic displacement
• Therefore, we can restrict k to within the first BZ [-π/a, π/a]
• Function with periodicity 2π/a
���� � 2�
�
v� � ��⁄ Phase velocity
Note that:
• In above equation n is cancelled out, this means that the eqn. of motion of allatoms leads to the same algebraic eqn. This shows that our trial function Un isindeed a solution of the eqn. of motion of n-th atom.
• We started from the eqn. of motion of N coupled harmonic oscillators (if oneatom starts vibrating it does not continue with constant amplitude, but transferenergy to the others)
• Our wavelike solutions are uncoupled oscillations called normal modes:each k has a definite ω given by above eqn. and oscillates independently of theother modes.
• the number of modes is expected to be the same as the number ofequations N.
G. Bracco-Material Science SERP CHEM 10
Monoatomic Chain
� = 4�� sin �
2
G. Bracco-Material Science SERP CHEM 11
Monoatomic Chain
For a finite chain of N atoms we have to
choose the boundary conditions:
Since the solution is a travelling wave, a
suitable condition is the
periodic boundary condition (PBC)
u0(t) = uN(t)
This conditions (generally in 3D) is called
Born–von Karman boundary condition
N=8
1 8
08
0
0
2, 1, 2
or 1,2 2
exp(
exp[ ( ,
)
)]
1n n
N
mk m N
N aN N
m
u A i kX t
u u ikNa
π
ω
∴ = =
= − +
== −= ⇒
L
L
The value of k is discrete
with spacing ∆k=2π/Na
Each k describes a normal
mode of the vibration
Pattern of vibration:
• k ~ 0, exp(ikXn) ~ 1.
Every atom move in unison→ Little restoring force.
• k ~ π/a,exp(ikXn) ~ (-1)n.
Adjacent atoms move in opposite directions→ Maximum restoring force.
( )( ) ,ni kX tn nu t Ae X naω−= =Displacement of the n-th atom
Velocities of wave (phase velocity, group velocity):
• k ~ 0, Linear dispersion ω = (ωM
a/2)k→ phase velocity = group velocity
This is the long wavelength condition for elastic sound wave
• k ~ π/a, group velocity ~ 0 → no transmission of energy
Bragg condition: in 1D, waves are reflected back (2θ=180°→θ=90°)
→ 2d sin(θ)=nλ → 2d=n(2π/k) at k= π/a→d=a, therefore at these points
Bragg condition is verified.
p
g
vkd
vdk
ω
ω
=
=
G. Bracco-Material Science SERP CHEM 12
Monoatomic Chain
G. Bracco-Material Science SERP CHEM 13
If k ∼0 →λ is very long and waves are not sensitive to
the lattice structure of the crystal → continuum
approximation of the material.
ka<<1 → sin(ka)≈ka →ω = (ωMax
a/2)k
Sound velocity Vs= (ω
Max a/2)=
�
�a
k
ωContinuum
Discrete
0
Monoatomic Chain
Or using macroscopic parameters Vs=�
�⁄=
��
�, � � density Kb=bulk modulus
Longitudinal Waves
Transverse Waves
Remember that in 1D
Wave polarization
in 2D and 3D
Physical significance of wave numbers outside [-π/a, π/a]?
x
un
un
xa
G. Bracco-Material Science SERP CHEM 14
Monoatomic Chain
But
�
�and +
�
�
�are not equivalent →
�
�- 2
�
�= -
�
�
�is equivalent →same pattern, ω, and velocity
ω
k
π/a-π/a
4λ=7a →λ=��
�→ ��
��/�=��
��
3λ=7a →λ=��
→ ��
��/=�
��
Equivalent vectors
�
�+ Gn =
�
�+ n
�
�n integer (-∞, …-2,-1,0,1,2,…,∞)
� there is only one possible propagation direction and one polarizationdirection → 1D crystal has only one sound velocity.
� In this calculation only nearest neighbor interaction although this is agood approximation for the inert-gas solids, its not a good assumption formany solids (depends on the range of interactions)
� Extending to a model beyond nearest neighbor interaction many of thefeatures in above calculation are preserved.
• Wave equation solution still satisfies.
• The detailed form of the dispersion relation is changed but ω is still periodic function of k with period 2π/a
• Group velocity vanishes at k=(±)π/a
• There are still N distinct normal modes
• Furthermore the motion at long wavelengths corresponds to sound waves
G. Bracco-Material Science SERP CHEM 15
Monoatomic Chain
2�
�
number of modulations = number of further
neighbor interactions to be considered
Lattice with basis: diatomic chain
• Two different types of atoms of masses M1 and M2 are connected by
identical springs of spring constant α;
Vn-1Un Vn Un+1 Vn+1
α α α αM2 M2M1
M2M1a)
b)
(n-1) (n) (n) (n+1) (n+1) cells
a
• This is the simplest possible model of an ionic crystal.• Since a is the periodicity, the nearest neighbors separations is a/2
G. Bracco-Material Science SERP CHEM 16
M2 M1 M2M1 M2
Vn-1Un Vn Un+1 Vn+1
Equation of motion for mass M1 (nth):mass x acceleration = restoring force
Equation of motion for mass M2 (nth):
G. Bracco-Material Science SERP CHEM 17
Diatomic chain
We will consider only the first neighbour interaction although it is a poorapproximation in ionic crystals because electrostatic interaction is a long rangeinteraction.
M1� = � �� − � + � ���� − � =� �� + ���� − 2�
M2�� = � ��� − �� + � � − �� =� ��� + � − 2��
2
1 12
2
2 12
( 2 ),
( 2 ).
nn n n
nn n n
d uM v v u
dt
d vM u u v
dt
α
α
−
+
= + −
= + − a
vn-1
un
vn
un+1
Diatomic chain
22
21
2 2 cos( / 2)
2 cos( / 2) 2det 0.
M ka
ka M
α ω αα α ω
⇒
− − − −
=
2 22
1 2 1 2 1 2
1 1 1 1 4sin ( / 2).
ka
M M M M M Mω α α±
+ + −
⇒ = ±
212
221
2 2 cos( / 2)
2 cos( / 2) 2 0,
AM ka
Aka M
α ω αα α ω
− − − −
⇒ =The 2nd grade equation gives
two solutions with different
dispersion relations ω(k)
1( 1/2)
2
Assume ikna
n i t
ik n an
u A e
v A ee ω−
+
=
We need two non trivial independent
solutions that are travelling waves
G. Bracco-Material Science SERP CHEM 18
Two branches of dispersion
curves: optical & acoustic
assume M2 > M1
Diatomic chain
Patterns of vibration:
similar
a
d
b
c
a
b
c
d
G. Bracco-Material Science SERP CHEM 19
G. Bracco-Material Science SERP CHEM 20
Diatomic chain
�2 =�(�
1+�
2)
�1�
2
1 ± 1 −�
1�
2)
2 �1+�
22 2�2
� 2�2
2(�1+�
2)
In long wavelength region (ka«1); sin(ka/2)≈ ka/2 in ω(k), using a Taylor expansion:
Optical modes: Atoms in the cell oscillate out of
phase (for k=0 the center of mass is at rest
M1un+M2vn=0)
����2 =
2�(�1+�
2)
�1�
2
��2 =
� 2�2
2(�1+�
2)
Acoustic modes: atoms in the cell oscillate with
a small phase difference.
The sound speed is
��= �
�
2(�1+�
2)
• The other limiting solutions of equation ω2 are for ka= π (k at BZ),
→ sin(ka/2)=1. In this case
G. Bracco-Material Science SERP CHEM 21
Diatomic chain
���2 =
�(�1+�
2) ± �(�
2−�
1)
�1�
2
����2 =
2�
�1
����2 =
2�
�2
Optical modes: light atoms in the cell oscillate out
of phase with other light atoms, heavy atoms are at
rest
Acoustic modes: heavy atoms in the cell
oscillate out of phase with other heavy atoms,
light atoms are at rest
• The bandwidth ����
− ����
of both modes depends on α: in particular,decreasing the coupling α the curve for optical mode tends to become flat(dispersionless modes)
2a
G. Bracco-Material Science SERP CHEM 22
a
If M1=M
2
• If M1<M
2
Change of Periodicity a →2a
Repeat the dispersion curve
with the new periodicity
• If M1<M
2
Opening of gaps
At the BZ boundary
Monotomic chain →Diatomic chain
k
in k
How many normal modes (k points) in each branch?
1( 1/2)
2
0
0
2, 1,2
or 1,2 2
Imposing PBC on
exp( ) 1
iknan i t
ik n an
N
N
u A e
v A e
u u
v v
mk m N
N aN N
m
ik
e
Na
ω
π
−+
∴ = =
= − +
=
= =⇒
L
L Same as before for the lattice with no basis
• The total number of k points is N, number of cells, the total Degrees
Of Freedom (DOF) is 2N equal to number of the atoms (this remains
true for complex crystals in higher dimensions)
Diatomic chain
G. Bracco-Material Science SERP CHEM 23
Modes for the diatomic chain
Amplitude of vibration is strongly exaggerated!
G. Bracco-Material Science SERP CHEM 24
Acoustic mode
Optical mode
• The acoustic branch has this name because it gives rise to long
wavelength vibrations which travel with the speed of sound and ω→0 fork→0. The 2 atoms generally vibrate with a small phase difference
• The optical branch is a higher energy vibration and they vibrate generally
in out of phase. If the two vibrating atoms are ions, a dipole is crated duringthe vibration that can interact with electromagnetic radiation giving opticalproperties to the crystal.
G. Bracco-Material Science SERP CHEM 25
Diatomic chain
to understand the energy difference between acoustic & optical modes
Let’s examine the oscillation pattern for acoustic modes
k→0 (long wave length) shorter wavelength
The phase difference between neighbors is generally small
Instead for optical modes
The difference is always very close to the out of phase
The spring are less compress in acoustic mode → lower energy for acoustic
branches
G. Bracco-Material Science SERP CHEM 26
Vibration of a 3D lattice
1x Longitudinal Waves
2x Transverse Waves
Wave polarization
In 3D the atomic displacement of the atom in a cell (l,m,n→R=la+mb+nc) has
3 components ����=(����, ����, ����) → R+u
The energy of interaction in harmonic approximation between atoms is given
by
V=�
∑ ������ � � � �� ����′�� where ��� � ����� �
���
�� � �������| !"
Equation of motion: M ����=�∑ ��� � � �� ����′��
assuming a sinusoidal solution , ��, ��� � ����#∙���%�, � polarization vector
the solutions are similar to 1D case but with 3 different polarizations
Sodium (BCC)
Monoatomic
Three dimensional vibration
Along a given direction of propagation, there are 1 longitudinal wave and 2
transverse waves, each may have different velocities,
• monoatomic systems have only acoustic branches
G. Bracco-Material Science SERP CHEM 27
28
Energy scales:
Wavenumber (cm-1): A wavelength of energy that is also called a reciprocal
centimeter. Wavenumbers are obtained when frequency is expressed in Hertz
and the speed of light is expressed in cm/s (c= 29979245800 cm/s).
Electron Volt (eV): The electron volt is the energy that we would give an
electron (e=1.60217657 10-19 C) if it were accelerated by one volt potential
difference. 1 eV = 1.602 x 10-19 J.
Frequency f and angular frequency ω: the vibrational energy is �� � ��con h
the Planck constant (h= 6.62606957 10-34 Js, � �&
�= 1.054571726 10-34 Js)
1 THz→��/� � ��/� �0.0041356 eV= 4.1356 meV
1 THZ→ (1 1012 Hz)/c =33.3564095 cm-1
Longitudinal Waves
Transverse Waves
Energy difference:
Longitudinal modes usually corresponds to a greater compression of the
springs with respect to transvers ones → higher energy
G. Bracco-Material Science SERP CHEM
G. Bracco-Material Science SERP CHEM 29
Neon (FCC) monoatomic
All modes are acoustic (Monoatomic system)
Transverse mode are degenerate along [001]
and [111]. The maximum frequency is lower
than that of sodium since van der Waals
interaction in neon is weaker than metal
bond in sodium.
along [110] the longitudinal mode presents a non monotonic behavior different from a
sinusiodal one: this suggests that at least a next near neighbor interaction is necessary
This is related to the FCC structure: atom A of plane I
interacts with nearest neighbor C atoms in plane II and with
next nearest neighbor B in plane III.
3D crystal with atom basis
For a 3-dim crystal, if each unit cell has p atoms, the degrees of
freedom (DOF) are 3p for each cell (polarization L, T1
e T2)
3 acoustic branches, 3(p-1) optical branches
• If a crystal has N unit cells, then each branch has
N normal modes (number of k-points for each dispersion curve).
• As a result, the total number of normal modes of the whole
crystal is 3pN (= total DOF of this crystal).
G. Bracco-Material Science SERP CHEM 30
P=2 atoms (Ga, As),
then there are
3 acoustic branches L, T1
e T2
3 optical branches L, T1
e T2
FCC lattice with 2-atom basis (diamond)
cm-1
K
∼9Thz
The covalent bond is stronger than previous bonds
therefore maximum frequency is higher.
Γ Is the center of the first BZ and X, K, W, and L
special points on BZ boundary.
3D crystal with atom basis
G. Bracco-Material Science SERP CHEM 31
G. Bracco-Material Science SERP CHEM 32
P=2 atoms (C, C),
then there are
3 acoustic branches L, T1
e T2
3 optical branches L, T1
e T2
Diamond FCC lattice with 2-atom basis (diamond)
∼39Thz
The covalent bond is even stronger than previous one (the strongest bond!)
G. Bracco-Material Science SERP CHEM 33
NaCl (Sodium Chloride structure)
The structure corresponds to two
interpenetrating FCC lattices
two atoms in the unit cell
→ p=2 (6 vibrational branches)
The lattice vibration spectrum shows
acoustic (A) and optical (O) modes with
longitudinal (L) and transverse (T)
polarization.
The stronger interaction determines a
maximum frequency higher than the
case of neon and sodium
G. Bracco-Material Science SERP CHEM 34
Even for the more complex
structure of α-alumina with 10
atoms in the primitive cell
(p=10, 30 vibrational branches),
there are 3 acoustic branches
and 27 optical branches
Conventional Hex.cell Primitive Trigonal cell
αααα-Al2O
3
G. Bracco-Material Science SERP CHEM 35
PBC or Born–von Karman boundary condition
The concept of periodic boundary condition can extended to 2D and 3D
1 8
Moreover any finite system can be considered as
part of an infinite system
1D: un(t) = un+N(t)
3D: ul,m,n(t) = ul+N1,m,n(t)
ul,m,n(t) = ul,m+N2,n(t)
ul,m,n(t) = ul,m,n+N3(t)
81
A finite 2D area is mapped on a torus
X: u0,n(t) = uM,n (t) M,N integers
Y: um,0(t) = um,N (t)
In 3D it is not possible to make a
graphical representationM cells
N c
ell
s