3.8 Gibbs Free Energy Worksheet - Piedra Vista High...
Click here to load reader
Transcript of 3.8 Gibbs Free Energy Worksheet - Piedra Vista High...

3.8 – Gibbs Free Energy – Worksheet
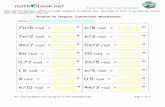
1. Calculate G using G = H - T S. Also, for each question, tell whether or not the reaction will be spontaneous.
a) CH3OH(l) + 1½ O2(g) CO2(g) + 2 H2O(g)
H = -638.4 kJ S = 156.9 J / K
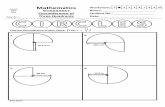
b) 2 NO2(g) N2O4(g)
H = - 57.2 kJ S = -175.9 J / K 2. Again find ΔG at 25°C for the reaction CH3CO2H (l) + 2 O2 (g) → 2 CO2 (g) + 2 H2O (g) This time using the Table of Thermochemical Data and the formula: ΔG = ΣΔG° products - ΣΔG° reactants 3. For the reaction below, Fe2O3 (s) + 3 CO (g) → 2 Fe (s) + 3 CO2 (s) ΔG° = -31.3 kJ Calculate the standard free energy of formation of the ferric oxide, Fe2O3. 4. Calculate ΔG at 25°C for the following reaction using ΔG = ΔH - T ΔS. Will this reaction be spontaneous at this temperature? CH3CO2H (l) + 2 O2 (g) → 2 CO2 (g) + 2 H2O (g)