Web view• Pauli exclusion principle, p. 239 • electron configuration, p. 240 • Hund's...
Transcript of Web view• Pauli exclusion principle, p. 239 • electron configuration, p. 240 • Hund's...

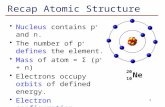
Electron Structure and Periodicity Chapter 6: Electron Structure of Atoms Chapter 7: Periodic Properties of the Elements
CHAPTER 6-7 OBJECTIVES • Understand the relationships c =λν and E = hν.
• Understand the concept of a quantized atom and its relationship to a line spectra of atoms. • Explain the concept of ionization energy.
• Describe the Uncertainty Principle and its affect on atomic theory. • Understand the relationship λ= h/mv and its affect on atomic theory.
• Describe how quantum numbers define electron orbitals and their value limitations. • Describe the shapes of the orbital types.
• Understand the concept of electron spin and how it relates to electron configuration. • Write the electron configuration both symbolically and as an orbital diagram for any element.
• Be able to write electron configurations, especially valence configurations, for any element, using the periodic table with the knowledge of the s,p,d, and f blocks.
• Describe the variations of atomic radii in the groups and periods on the periodic table and the underlying reasons for the variations.
• Describe and explain the observed changes in successive ionization energies for a given atom. • Describe the variations in first ionization energies in the groups and periods on the periodic table and the underlying
reasons for the variations. • Do the same with the electron affinities of the elements.
• Describe the periodic trends in metallic and nonmetallic behavior and chemical activity. • Electron Energy Levels and Quantum Mechanics
• Electron Configurations and the Periodic Table • Periodic Relationships: Atomic Radii, Ionization Energy, Electron Affinity, and Oxidation State
Key Words: • electronic structure, p. 217 • electromagnetic radiation, p. 218
• wavelength, p. 218 • frequency, p. 218
• quantum, p. 221 • Planck's constant, p. 221
• photoelectric effect, p.222 • photon, p. 222
• spectrum, p. 224 • continuous spectrum, p. 224
• line spectrum, p. 225 • ground state, p. 226
• excited state, p. 226
• orbital, p.232
• quantum numbers, p. 232 • electron shell, p. 233
• subshell, p. 233 • degenerate orbitals, p. 239
• Pauli exclusion principle, p. 239 • electron configuration, p. 240
• Hund's rule, p. 243 • core electrons, p. 244
• valence electrons, p. 244 • effective nuclear charge, p. 263
• transition elements, p. 244 • atomic radius, p. 267
• isoelectronic series, p. 269
• ionization energy, p. 271
• electron affinity, p. 275 • metallic character, p. 277
• metal, p. 277 • nonmetal, p. 279
• metalloid, p. 281 • alkali metal, p. 281
• alkaline earths, p. 281 • hydride ion, p. 282
• halogens, p. 288 • noble gases, p. 289
Troubleshooting Tips/Error Traps: • Orbitals are areas of highest probability of finding the electron, not the path of the electron. • Electron shells in an atom are diffuse and overlap considerably.
• Electron affinity is not the opposite of ionization energy.

STUDY GUIDE - Chapter 8/9 Exam
Bonding in Ionic Compounds - (Review electron configuration notation)Chemical Bonds - Ionic and Covalent Bonds - What is the difference in these types of bonds? Which elements typically form an ionic bond? a covalent bond? The Lattice Energy - To which type of bond does this apply? Failure of the Octet Rule - Which elements always obey the Octet Rule? Why? Which elements can exceed the octet? Which elements don't need to have an octet?Lewis Symbols Can you write them?The Covalent Bond - Do you know the definition? Bond Energy, Length and Distance - What is the association between these concepts? Single, Double & Triple Bonds - How many electrons are shared in these bonds? Exceptions to the Octet Rule - Why?Bond Energies – Know how to calculate the Enthalpy of a reaction with bond energies.Bond Order and Some Bond Properties - Do you know the definition of Bond Order? Do you know the relationship between bond order, type of bond, bond length, bond energy and bond frequency?Resonance - How do we know this occurs? What is meant by the average bond order?Modified Lewis Structures for Resonance Hybrids - Can you draw them?Formal Charge and the Selection of Lewis Structures: Why do we use it? Can you compute the formal charge of different structures? If there are different possible structures, which one is the preferred structure? Why?Coordinate Covalent Bonds - Can you think of an example?Polar Molecules and Electronegativity Pauling's Scale & Periodic Trends - Do you know the periodic trend?Which are the electropositive elements? What makes a molecule polar? Can you write the delta notation? If there is a difference in electronegativity at what point does a bond change from a polar covalent bond to an ionic bond? Do you know the difference between electronegativity and electron affinity? Do you know the difference between a pure covalent, polar covalent and an ionic bond?VSEPR, Electron Pair Geometry and Molecular Geometry - Know the names, shapes, angles and VSEPR formula of each of the electron pair geometries and molecular geometries of compounds and polyatomic ions. Can you draw the Lewis Structure of a compound, determine its electron pair geometry, its molecular geometry and the angles and names associated with each? Polarity - Be able to predict the bond polarity and the overall polarity of a species based on its geometry and the electronegativity of its substituents. Do you remember the electronegativity trend on the periodic table? Hybrid Orbitals - Know the number of hybrid orbitals possible for each type of hybrid. How many orbitals does an sp2 hybrid possess? Know the molecular shape of each type of hybridization and the angles associated with this shape. Be able to predict the hybridization for a species (compound or polyatomic ion). Can you write the orbital notation? Do you know what MX2E1 represents? See homework worksheet.Sigma and Pi bonds - Know the type of overlap, the strength of each bond type, how many and of which type are present in single, double, and triple bonds, and which type of orbitals are used to form them as well as which elements are most likely to form these kinds of bonds. Can you name any elements that cannot possibly form a pi bond? Which bond always forms first? Why can't atoms at the bottom of the periodic table form pi bonds?
Key Terms: chemical bonds, ionic bonds, covalent: pure, nonpolar, polar, coordinate, metallic bonds, Lewis symbols, octet rule, lattice energy, electronegativity, dipole, polar molecule, formal charge, resonance structure, bond enthalpy, VSEPR, lone pair, electron pair geometry (aka electron-domain geometry, molecular geometry, bond dipole, hybrid orbital, sigma and pi bonding, delocalized pi bonding, paramagnetism, diamagnetism, linear, trigonal planar, tetrahedral, trigonal bipyramidal, octahedral, linear, bent, trigonal pyramidal, t-shaped, square planar, all their bond angles (review the Molecular Geometry Lab Sheet).