Unit 7
description
Transcript of Unit 7

Unit 7Unit 7Nuclear ChemistryNuclear Chemistry
ByByKevin “KG” Gerber Ding and Kevin “KG” Gerber Ding and
Steven “The Flash” HougSteven “The Flash” Houg

Radioactive DecayRadioactive Decay
α-particleββ -particle -particleγγ-ray-ray

The Alpha ParticleThe Alpha Particle
• α or He• Largest of the 3 radioactive
particles• Lowest penetrating ability Sample Decay:Sample Decay:
U U α + Th
42
42
42
23592
23190

The Beta ParticleThe Beta Particle
oror Same electrical charge and Same electrical charge and
mass as an electronmass as an electron Sample Decay:Sample Decay:
N N OO + + ee
e 0-1
0-1β
0-1
13 8
13 7

Gamma RaysGamma Rays
YY
Gamma rays are photonsGamma rays are photons They have no mass and no They have no mass and no
chargecharge
00

Positron EmissionPositron Emission
oror Positrons are equivalent to Positrons are equivalent to
positively charged electrons or positively charged electrons or positively charged beta particlespositively charged beta particles
Formed when a neutron splits Formed when a neutron splits into a neutron and another into a neutron and another particle (positron)particle (positron)
p e + np e + n
0+1e β 0
+1
0+1
10
11

Electron CaptureElectron Capture
Occurs when an electron is captured Occurs when an electron is captured by the nucleusby the nucleus
e + p ne + p n 0 -1
1+1
10

Radioactive Decay SeriesRadioactive Decay Series
238 92U 4
2α +234 90T
h+β 0
-1234 91Pa +β 0
-1234 92U +4
2α
234 90T
h234 91Pa234 92U230 90T
h

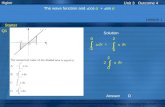
Half Life EquationHalf Life Equation
N=NN=N00ee-kt-kt
K=K= ln 2 T1/2


Theory of RelativityTheory of Relativity
∆∆E= (∆m)cE= (∆m)c22
C is the speed of light (2.9*10C is the speed of light (2.9*1088 m/s m/s22)) m is mass (kg)m is mass (kg) E is energy (Joules)E is energy (Joules)