Small Interfering RNA Knockdown of Calcium-independent Phospholipases A2 ² or ³ Inhibits the
RNA interference-mediated knockdown of Notch-1 inhibits migration and invasion, down-regulates...
Transcript of RNA interference-mediated knockdown of Notch-1 inhibits migration and invasion, down-regulates...

A
Ras
Ya
b
c
a
ARRAA
KNsPTCC
I
nhs2wmidflrc2
HR
h0
ARTICLE IN PRESSG ModelCTHIS-50843; No. of Pages 9
Acta Histochemica xxx (2014) xxx–xxx
Contents lists available at ScienceDirect
Acta Histochemica
jo ur nal homepage: www.elsev ier .de /ac th is
NA interference-mediated knockdown of Notch-1 inhibits migrationnd invasion, down-regulates matrix metalloproteinases anduppresses NF-�B signaling pathway in trophoblast cells
ang Yua, Leilei Wanga, Weiwei Tangb, Dan Zhangc, Tao Shanga,∗
Department of Obstetrics and Gynecology, Shengjing Hospital, China Medical University, Shenyang 110004, People’s Republic of ChinaDepartment of Gynecology, Liaoning Cancer Hospital and Institute, Shenyang 110042, People’s Republic of ChinaDepartment of Obstetrics and Gynecology, The Women and Infants Hospital of Shenyang City, Shenyang 110014, People’s Republic of China
r t i c l e i n f o
rticle history:eceived 21 January 2014eceived in revised form 4 March 2014ccepted 5 March 2014vailable online xxx
eywords:otch-1
hRNAreeclampsia
a b s t r a c t
Preeclampsia is well known to present with reduced trophoblast invasion into the placental bed. Notch-1, a ligand-activated transmembrane receptor, has been reported to be down-regulated in preeclamptichuman placentas. This study was conducted to explore the role of Notch-1 in the cell migration andinvasion of a human trophoblast cell line, JEG3 cells. Short hairpin RNA (shRNA)-mediated RNA inter-ference was performed to effectively suppress the endogenous expression of Notch-1 at both mRNAand protein levels in JEG3 cells. Results of wound healing and transwell assays showed that knockdownof Notch-1 reduced trophoblast cell migration and invasion. The protein expressions and activities ofmatrix metalloproteinase (MMP)-2 and MMP-9 were reduced in JEG3 cell when Notch-1 was decreased.Furthermore, the epithelial-cadherin (E-cadherin) expression increased in JEG3 cells when Notch-1 was
rophoblast cellsell migrationell invasion
inhibited, whereas its suppressor Snail decreased in these cells. Moreover, knockdown of Notch-1 alsosuppressed NF-�B signaling pathway by inhibiting the phosphorylation of nuclear factor kappa B (NF-�Bp65) inhibitor (I�B�) and the subsequent nuclear translocation of NF-�B subunit p65 in JEG3 cells. Insummary, we demonstrate that Notch-1 contributes to trophoblast cell migration and invasion and thatit may be involved in the pathology of human preeclampsia.
ntroduction
Preeclampsia is a pregnancy disorder of the second half of preg-ancy, labor, or the early period after delivery characterized byypertension, abnormal amounts of protein in the urine, and otherystemic disturbances (Redman and Sargent, 2005; Winn et al.,011). This condition complicates 5–8% of all pregnancies world-ide and increases both maternal and neonatal morbidity andortality (Kanasaki and Kalluri, 2009; Hansson et al., 2013). Dur-
ng pregnancy, the specialized placental cells called trophoblastsifferentiate and invade the uterus, establishing maternal bloodow to the placenta (Lain and Roberts, 2002). However, earlier
Please cite this article in press as: Yu Y, et al. RNA interference-medown-regulates matrix metalloproteinases and suppresses NF-�B sighttp://dx.doi.org/10.1016/j.acthis.2014.03.003
eported studies have demonstrated that placental trophoblastells fail to invade the uterus properly in preeclampsia (Fisher,004; McMaster et al., 2004). Although preeclampsia has been
∗ Corresponding author at: Department of Obstetrics and Gynecology, Shengjingospital, China Medical University, 36 Sanhao Street, Shenyang 110004, People’sepublic of China.
E-mail address: [email protected] (T. Shang).
ttp://dx.doi.org/10.1016/j.acthis.2014.03.003065-1281/© 2014 Elsevier GmbH. All rights reserved.
© 2014 Elsevier GmbH. All rights reserved.
recognized to cause poor placentation during pregnancy, relativelylittle is known about its pathogenesis.
Notch-1 is a ligand-activated transmembrane receptor whichgoverns differentiation and function during cell–cell contact inmany tissues (Artavanis-Tsakonas et al., 1999; Bianchi et al., 2006).Ample evidence has shown that Notch-1 mediates migration andinvasion of different tumor cell types, including pancreatic cancercells (Wang et al., 2006), ovarian carcinoma cells (Sahlgren et al.,2008) and prostate cancer cells (Wang et al., 2010a). Notably, pla-cental trophoblast cells acquire tumor-like properties that allowthe cells to invade the uterus and extensively remodel the maternalvasculature (Fisher, 2004; Winn et al., 2009). A study from Cobelliset al. (2007) shows that Notch-1 is down-regulated in preeclamp-tic placentas as compared with normal physiological placentas(Cobellis et al., 2007), suggesting Notch-1 may be involved in thepathology of human preeclampsia. Based on the above reportedfacts, we hypothesized that Notch-1 may participate in the pathol-
diated knockdown of Notch-1 inhibits migration and invasion,naling pathway in trophoblast cells. Acta Histochemica (2014),
ogy of preeclampsia by mediating the migration and invasion oftrophoblasts in the placenta.
In this study, we therefore investigated the role of Notch-1 inpreeclampsia with emphasis of its effects on trophoblast migration

ING ModelA
2 chemi
awh
M
C
iR1l
P
usdpfsGasggimwstvr
loCr
Q
(r(cl2sNP3(C
W
adn1b
ARTICLECTHIS-50843; No. of Pages 9
Y. Yu et al. / Acta Histo
nd invasion. Short hairpin RNA (shRNA) interfering technologyas performed to inhibit the endogenous expression of Notch-1 inuman JEG3 trophoblast cells of the placenta.
aterials and methods
ell culture
Human choriocarcinoma JEG3 cells were obtained from Amer-can Type Culture Collection (Manassas, VA, USA) and cultured inPMI 1640 medium (Gibco BRC, Paisley, UK) containing 10% FBS,00 U/mL penicillin and 100 �g/mL streptomycin (Gibco BRC, Pais-
ey, UK) at 37 ◦C and 5% CO2.
lasmid construction and cell transfection
The pGCsi-H1 vector (GeneChem, Shanghai, China) wassed to generate shRNAs targeting Notch-1 (Genbank Acces-ion: NM 017617.3). The transfection efficiency was tracked viaetecting the green fluorescent protein (GFP) co-expressed byGCsi-H1 vector. Two pairs of oligonucleotides encoding shRNAsor Notch-1 were designed and synthesized as follows: Notch-1hRNA-1 (forward, 5′-GATCCCCctgcgagatcaacctggatTTCAAGA-AatccaggttgatctcgcagTTTTT-3′; reverse, 5′-AGCTAAAAActgcg-gatcaacctggatTCTCTTGAAatccaggttgatctcgcagGGG-3′), Notch-1hRNA-2 (forward, 5′-GATCCCCtccaacccttgtgtcaacgTTCAAGAGAc-ttgacacaagggttggaTTTTT-3′; reverse, 5′-AGCTAAAAAtccaaccctt-tgtcaacgTCTCTTGAAcgttgacacaagggttggaGGG-3′). The sequencesn lowercase indicated two insert sequences targeting Notch-1
RNA (1911–1929 bp and 2275–2293 bp). These oligonucleotidesere then annealed and sub-cloned into the HindIII and BamHI
ites of the pGCsi-H1 vector. Meanwhile, negative control shRNAargeting scrambled sequence was also inserted into pGCsi-H1ector. The accuracy of the recombinant vectors was confirmed byestriction enzyme analysis and sequencing.
For plasmid transfection, JEG3 cells of confluent cell mono-ayer were transiently transfected with Notch-1 shRNA plasmidsr control vector using Lipofectamine 2000 (Invitrogen, Carlsbad,A, USA) according to the manufacturer’s instructions. Cells onlyeceiving mock transfection served as normal controls.
uantitative real-time PCR
Total RNAs were isolated from cells using Trizol reagentInvitrogen), and cDNAs were synthesized using Super M-MLVeverse transcriptase kit (BioTeke, Beijing, China). SYBR GreenSolarbio, Beijing, China) was used for real-time PCR on Exicy-lerTM 96 (Bioneer, Daejeon, Korea), and the mRNA expressionevels of Notch-1 were quantified via 2−��CT (Muller et al.,002). Analytical data were normalized to the mRNA expres-ion level of endogenous control �-actin (Genbank Accession:M 001101.3). The primers used for quantitative real-timeCR were Notch-1 (forward 5′-CTGCCCGTTCTTGAAATGTAGG-′ and reverse 5′-TGTTGCTGGAGCATCTTCTTCG-3′), and �-actinforward 5′-CTTAGTTGCGTTACACCCTTTCTTG-3′ and reverse 5′-TGTCACCTTCACCGTTCCAGTTT-3′).
estern blot
Protein samples from cell lysates were subjected to Western blot
Please cite this article in press as: Yu Y, et al. RNA interference-medown-regulates matrix metalloproteinases and suppresses NF-�B sighttp://dx.doi.org/10.1016/j.acthis.2014.03.003
nalysis using rabbit monoclonal antibodies for Notch-1 (1:1000iluted; Cell Signaling Technology, BSN, MA, USA), phosphorylateduclear factor kappa B (NF-�B) inhibitor alpha (phosphor-I�B�;:1000 diluted; Cell Signaling Technology), rabbit polyclonal anti-odies for epithelial-cadherin(E-cadherin; 1:1000 diluted; Bioss,
PRESSca xxx (2014) xxx–xxx
Beijing, China), Snail (1:500 diluted; Abcam, Cambridge, UK),matrix metalloproteinase-2 (MMP-2; 1:100 diluted; Santa CruzBiotechnology, Santa Cruz, CA, USA), MMP-9 (1:1000 diluted;Bioss), total I�B� (1:100 diluted; Cell Signaling Technology). �-Actin was used as endogenous control. For detecting nucleartranslocation of NF-�B p65 subunit, nuclear extracts were firstprepared via Nuclear and Cytoplasmic Protein Extraction Kit (Bey-otime, Shanghai, China) and then subjected to Western blotanalysis using rabbit polyclonal antibody against p65 (1:100diluted; Santa Cruz Biotechnology), with lamin A as nuclear con-trol (Onai et al., 2007). In brief, protein samples were extractedusing RIPA lysis buffer (Beyotime) and then resolved in sodiumdodecyl sulfate-polyacrylamide gel electrophoresis and trans-ferred to polyvinylidene fluoride membranes. The membraneswere incubated with appropriate antibodies, washed and incu-bated with horseradish peroxidase-coupled secondary antibodies.Protein blots were detected using the ECL-Plus Western blottingdetection system.
Gelatin zymography
Gelatin zymography assay was performed to determine theactivities of MMP-2 and MMP-9 in the supernatant of cells as previ-ously described (Toth et al., 2012). Briefly, the culture media werecollected, centrifuged, and then subjected to electrophoresis on10% acrylamide gel containing 1 mg/mL gelatin. Following elec-trophoresis, the gels were rinsed with 2.5% Triton X-100 (Amresco,Solon, OH, USA) at room temperature for 30 min and then incu-bated in 0.05 M Tris–HCl buffer containing 5 mM CaCl2 and 0.2 MNaCl at 37 ◦C for 16 h. After incubation, the gels were then stainedwith Coomassie brilliant blue R-250 (Amresco) for 3 h. The banddensities were analyzed using a scanning densitometer.
Methyl thiazolyl terazolium (MTT) assay
Cell viability was evaluated by colorimetric assay using MTT.Cells were dispensed at a density of 3 × 104 cells/well in 96-wellplates with complete RPMI1640 culture media. After various inter-vals of incubation, MTT reagent (Sigma–Aldrich, St. Louis, MO, USA)was added into each well to a final concentration of 0.2 mg/mL andincubated for 4 h at 37 ◦C. After centrifugation, MTT reagent wasgently removed. The formazan crystals were dissolved in DMSOand absorbance was read at 490 nm.
Wound healing assay
Cells in 6-well plates were first subjected to serum starvationfor 4 h at 20 h post-transfection. Then, a sterile 200-�L pipette tipwas used to make a straight scratch in cell monolayer carefully,simulating a wound. Thereafter, the wounded cell monolayer wasrinsed twice with serum-free medium gently and allowed to healin complete culture medium for 24 h. The width of the wound fieldwas photographed using an inverted microscope at 0, 12 and 24 hafter wound formation, respectively. Cell migration was assessedby determining the percent closure.
Matrigel invasion assay
Matrigel invasion assay were performed using Matrigel-coatedtranswell inserts containing polycarbonate filters with 8 �m pores(Corning Inc., Life Sciences, Tewksbury, MA, USA) according to man-
diated knockdown of Notch-1 inhibits migration and invasion,naling pathway in trophoblast cells. Acta Histochemica (2014),
ufacturer’s instructions. Briefly, the inserts were coated with 20 �LMatrigel (1 mg/mL; BD Biosciences, San Jose, CA, USA) and allowedto solidify for 2 h at 37 ◦C in a humidified atmosphere (5% CO2/95%air). After 24 h shRNA transfection, JEG3 cells (5 × 104) in 200 �L

ARTICLE IN PRESSG ModelACTHIS-50843; No. of Pages 9
Y. Yu et al. / Acta Histochemica xxx (2014) xxx–xxx 3
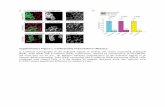
F ) ReprG exprw ificana s endo
sbltctcw
E
4tcp
S
S
ig. 1. Knockdown of Notch-1 expression using specific shRNAs in JEG3 cells. (AFP-positive cells were observed under fluorescent microscope. Notch-1 (B) mRNAere determined by Western blot analysis in JEG3 cells. The designed shRNAs sign
re presented as mean ± standard deviation (SD) (n = 3 per group). �-Actin served a
erum-free RPMI-1640 medium were plated onto the upper cham-ers, whereas 800 �L complete culture medium was added to the
ower wells. Twenty-four hours later, cells on the upper surface ofhe filter were gently removed by a cotton swab, and the invadedells below the filter were fixed in paraformaldehyde for 20 min andhen stained with hematoxylin for 3 min. The numbers of invadedells were then counted in each of five random areas for a givenell under a light microscope (200×).
nzyme-linked immunosorbent assay (ELISA)
Culture media of cells was collected and subjected to ELISA after8 h transfection. Protein levels of MMP-2 and MMP-9 were quan-ified by ELISA kits (USCN Life Science, Wuhan, China). The cytokineontents were expressed as pictograms (pg) per milligram (mg) ofrotein.
Please cite this article in press as: Yu Y, et al. RNA interference-medown-regulates matrix metalloproteinases and suppresses NF-�B sighttp://dx.doi.org/10.1016/j.acthis.2014.03.003
tatistical analysis
All data were presented as the mean ± standard deviation (SD).tatistical analysis was carried out by one-way ANOVA analysis,
esentative images of JEG3 cells that were transfected with shRNA plasmids. Theession levels were determined by real-time PCR and (C) protein expression levelstly reduced Notch-1 expression at both mRNA and protein level in JEG3 cells. Datagenous control. ***P < 0.001 versus control shRNA cells. Scale bar = 100 �m.
and Bonferroni post hoc test was used for multiple comparisons. AP value less than 0.05 was considered statistically significant.
Results
Knockdown of Notch-1 expression by plasmid-mediatedshRNA in JEG3 cells
To explore the role of Notch-1 in preeclampsia, shRNA-mediatedRNA interfering approach was performed to suppress Notch-1expression in human placenta-derived JEG3 trophoblastic cells. Wenoted that the percentage of GFP-positive cells was almost 100%in cells transfected with shRNA plasmids (Fig. 1a) at 48 h aftertransfection, suggesting that the designed shRNAs were success-fully expressed in these cells. Then, we evaluated the knockdownefficiencies of the two Notch-1 shRNAs in JEG3 cells using real-timePCR and Western blot analysis (n = 3 per group). At 48 h post-
diated knockdown of Notch-1 inhibits migration and invasion,naling pathway in trophoblast cells. Acta Histochemica (2014),
transfection, the mRNA (Fig. 1b) and protein (Fig. 1c) expressionlevels of Notch-1 in cells transfected with Notch-1 exclusive shRNAplasmids significantly decreased, as compared with the cells trans-fected by control vector. The above results indicate that the Notch-1

ARTICLE IN PRESSG ModelACTHIS-50843; No. of Pages 9
4 Y. Yu et al. / Acta Histochemica xxx (2014) xxx–xxx
Fig. 2. Knockdown of Notch-1 inhibits JEG3 cell migration and invasion. (A) Cell proliferation determined by MTT and presented as the absorbance values at 490 nm (n = 5per group). (B) Wound healing assay of JEG3 cells (n = 3 per group). Confluent cells first received transient transfection of different shRNA plasmids. Twenty-four hours later,a te tipi and thc scope*
st
Ki
M
straight scratch in cell monolayer was carefully created by a sterile 200-�L pipetnvasion (n = 3 per group). JEG3 cells were transfected with shRNA vectors for 24 h,ells were counted in each of five random areas for a given well under a light micro**P < 0.001 versus control shRNA cells. Scale bar = 100 �m.
pecific shRNAs can effectively suppress Notch-1 expression at bothranscriptional and translational levels.
nockdown of Notch-1 reduced JEG3 cell migration and
Please cite this article in press as: Yu Y, et al. RNA interference-medown-regulates matrix metalloproteinases and suppresses NF-�B sighttp://dx.doi.org/10.1016/j.acthis.2014.03.003
nvasion
To eliminate the interference induced by cell proliferation,TT analysis was conducted before cell migration and invasion
, and images were taken at 0, 12 and 24 h, respectively. (C) Transwell assay of cellen subjected to Transwell assay. Twenty-four hours later, the numbers of invaded
(200×). Data are presented as mean ± standard deviation (SD). *P < 0.05, **P < 0.01,
assessments. We noted that knockdown of Notch-1 inhibited JEG3cell proliferation after 48 h. However, no evident difference wasfound in proliferation between cells transfected with control shRNAand those treated with specific Notch-1 shRNAs at 12 or 24 h
diated knockdown of Notch-1 inhibits migration and invasion,naling pathway in trophoblast cells. Acta Histochemica (2014),
(Fig. 2a). Next, we performed wound healing assay at 0, 12 and24 h and transwell assay at 24 h after shRNA transfection in JEG3cells. Results showed a significant inhibition of cell migrationin the wounded area of cells transfected with Notch-1 shRNAs,

ARTICLE IN PRESSG ModelACTHIS-50843; No. of Pages 9
Y. Yu et al. / Acta Histochemica xxx (2014) xxx–xxx 5
Fig. 3. Inhibition of Notch-1 expression down-regulated the expression levels of MMP-2 and MMP-9 in cell lysates and culture medium of JEG3 cells. Cells were transfectedwith shRNAs for 48 h, and then subjected to determine the protein levels of MMP-2 and MMP-9 in cell lysates (A) using Western blot and in cultured medium (B) usingE gelatM tanda*
aMtiia
Ka
itetatWb
LISA. The activities of MMP-2 and MMP-9 in cell supernatant were determined byMP protein expression and activity in JEG3 cells. Data were presented as mean ± s
*P < 0.01, ***P < 0.001 versus control shRNA cells.
s compared with the control plasmid-transfected cells (Fig. 2b).eanwhile, the results of transwell invasion assay showed that
he invasive capacity of Notch-1-knowdown cells reduced signif-cantly as compared with control cells (Fig. 2c). The present datallustrate that knockdown of Notch-1 can reduce the cell migrationnd invasion of human trophoblastic cells.
nockdown of Notch-1 decreased MMP expression andctivity
MMPs are metal-dependent endopeptidases capable of degrad-ng extracellular matrix, and appear to play a critical role inrophoblast invasion (Zhu et al., 2012; Zhang et al., 2013). Toxplore the effects of Notch-1 on MMPs, we therefore documentedhe protein levels of MMP-2 and MMP-9 in cell lysates as well
Please cite this article in press as: Yu Y, et al. RNA interference-medown-regulates matrix metalloproteinases and suppresses NF-�B sighttp://dx.doi.org/10.1016/j.acthis.2014.03.003
s in culture medium at 48 h after transfection. Furthermore,heir activities in cell supernatant were also been determined.
hen compared with control plasmid transfected cells, Westernlot analysis showed that treatment of JEG3 cells with Notch-1
in zymography assay (C). Knockdown of Notch-1 induced significant decreases ofrd deviation (SD) (n = 3 per group). �-Actin served as endogenous control. *P < 0.05,
specific shRNAs clearly decreased the MMP-2 and MMP-9 levels incell lysates (Fig. 3a). Likewise, inhibition of Notch-1 caused a reduc-tion in the amounts of MMP-2 and MMP-9 in cultured medium(Fig. 3b). Moreover, results from gelatin zymography showed thatthe activities of MMP-2 and MMP-9 were reduced in cells whenNotch-1 was inhibited (Fig. 3c). These data suggest that Notch-1may regulate the trophoblast invasion of human placenta by medi-ating MMP expression and activity.
Knockdown of Notch-1 up-regulated E-cadherin expressionbut down-regulated Snail expression in JEG3 cells
E-cadherin is a cell–cell adhesion protein, and its abnor-mal expression is believed to be associated with preeclampsia(Kokkinos et al., 2010; Canel et al., 2013). Therefore, given the
diated knockdown of Notch-1 inhibits migration and invasion,naling pathway in trophoblast cells. Acta Histochemica (2014),
important role of E-cadherin in the differentiation of the trophecto-derm in animal embryo (Getsios et al., 2000), the protein expressionlevels of E-cadherin and its inhibitor Snail in JEG3 cells treatedwith different shRNAs were determined by Western blot at 48 h

ARTICLE ING ModelACTHIS-50843; No. of Pages 9
6 Y. Yu et al. / Acta Histochemi
Fig. 4. Suppression of Notch-1 expression increased E-cadherin expression anddecreased Snail in JEG3 cells. Cells were transfected with shRNAs for 48 h, and thensubjected to Western blot analysis to determine the endogenous protein expres-sion levels of E-cadherin and its inhibitor Snail. Increased E-cadherin and decreasedSac
aw(cN
Is
t2cpnbaTm
D
t2i
nail were observed in cells when Notch-1 was down-regulated. Data are presenteds mean ± standard deviation (SD) (n = 3 per group). �-Actin served as endogenousontrol. **P < 0.01, ***P < 0.001 versus control shRNA cells.
fter transfection. Data showed that E-cadherin increased in cellshen Notch-1 was inhibited, whereas Snail decreased in these cells
Fig. 4). Such findings indicate the involvement of increased E-adherin in reduced trophoblast migration and invasion caused byothch-1 inhibition.
nhibition of Notch-1 expression suppressed NF-�Bignaling pathway in JEG3 cells
Since NF-�B signaling pathway is reported to be associated withhe invasive process of various cancer cell types (Hagemann et al.,005; Wu et al., 2009), we explored whether knockdown of Notch-1ould affect this intracellular signal transduction by detecting I�B�hosphorylation and NF-�B p65 nuclear translocation in vitro. Weoted that, when compared with control shRNA treated cells, inhi-ition of Notch-1 markedly reduced I�B� phosphorylation (Fig. 5a)nd inhibited NF-�B p65 nuclear translocation (Fig. 5b) in JEG3 cells.hese results imply that inhibition of Notch-1 may reduce JEG3 celligration and invasion by suppressing NF-�B signaling pathway.
iscussion
Please cite this article in press as: Yu Y, et al. RNA interference-medown-regulates matrix metalloproteinases and suppresses NF-�B sighttp://dx.doi.org/10.1016/j.acthis.2014.03.003
The placenta is a transient organ that forms during pregnancyo support the growth and development of the fetus (Anin et al.,004). Abnormal placental development, especially the limited
nvasion of trophoblast cells into the uterus and the subsequent
PRESSca xxx (2014) xxx–xxx
failure of the remodeling of maternal spiral arteries, often causespreeclampsia, a pregnancy related pathology (Ji et al., 2013).Notably, a marked expression decrease of Notch-1 has been foundin preeclamptic placentas as compared with normal placentasduring the third trimester (Cobellis et al., 2007), indicating theinvolvement of Notch-1 in the pathology of human preeclampsia.Moreover, trophoblast cells physiologically invade into the uterusduring pregnancy, and this invasive behavior of trophoblasts is sim-ilar to tumor cells in malignancies (Zhou et al., 2002; Fitzgeraldet al., 2008). Considerable evidence has shown that Notch-1 con-tributes to the tumorigenesis of diverse cancers, including breastcancer (Parr et al., 2004), pancreatic cancer (Krausch et al., 2013),colon cancer (Zhang et al., 2010) and cervical cancer (Song et al.,2008), probably by regulating cancer cell growth, migration andinvasion (Wang et al., 2010b). These previous findings lead to ahypothesis that the aberrant expression of Notch-1 may be involvedin the pathological changes of preeclampsia, probably by mediatingtrophoblast cell migration and invasion. JEG3 cell line, derived froma human choriocarcinoma, secretes human leukocyte antigen-G(HLAG), human chorionic gonadotropin (hCG), placental lactogen,etc. (Kovats et al., 1990; Hannan et al., 2010). It is now widely usedas an in vitro model for studies on trophoblast invasion (Mandl et al.,2006; Ferretti et al., 2007). Here we demonstrated that inhibitionof Notch-1 via shRNA could reduce the migration and invasion inJEG3 cells, corresponding to prior researches conducted in pancre-atic cancer cells (Wang et al., 2006) and prostate cancer cells (Wanget al., 2010a). These findings suggest that Notch-1 may contributeto the invasive capability of placental trophoblasts.
MMPs are zinc-dependent endopeptidases that play importantroles in the control of diverse cellular processes (Visse and Nagase,2003; Nagase et al., 2006) and they have been reported be asso-ciated with the invasive progress of various cell types (Xiao et al.,2012; Zhu et al., 2012), including trophoblasts (Bischof et al., 1995).Therefore, we determined the effects of Notch-1 on the matrix met-alloproteases in placenta in vitro, with emphasis on MMP-2 andMMP-9. A prior study has shown that the expressions of both MMP-2 and MMP-9 in preeclamptic placentas are significantly declinedas compared with those in normal term placentas (Li et al., 2007),indicating a strong association between the decreased MMP-2 andMMP-9 and the occurrence of preeclampsia in human placentas.In support of this previous finding, we found that the reducedJEG3 cell migration and invasion were accompanied with remark-able decreases in MMP-2 and MMP-9 expressions and activities.Furthermore, knockdown of Notch-1 via small interfering RNAhas been demonstrated to inhibit endothelial cell migration andinvasion, and MMP-2 and MMP-9 activities (Gao et al., 2012), corre-sponding to our present results. Collectively, these studies suggestthat Notch-1 contributes to MMP-2 and MMP-9 related placentaltrophoblast invasion.
The placental trophoblast cells change from a well organizedcoherently layered phenotype to a migratory phenotype duringpregnancy, which resembles other developmental epithelial-mesenchymal transitions (EMTs) (Vicovac and Aplin, 1996).However, EMT in trophoblasts is inhibited in preeclamptic placen-tas (Kokkinos et al., 2010). Thus, we investigated whether inhibitionof Notch-1 could affect EMT process in the placenta by detectingthe expression levels of EMT regulator E-cadherin in vitro. Up-regulation of E-cadherin often inhibits the cell invasion (Zhao et al.,2012; Tripathi et al., 2013), whereas the loss of E-cadherin facili-tates EMT (Tian et al., 2011). Here we found that the protein levelsof E-cadherin were up-regulated in JEG3 cells when Notch-1 wasinhibited, whereas its direct inhibitor Snail (Cano et al., 2000) was
diated knockdown of Notch-1 inhibits migration and invasion,naling pathway in trophoblast cells. Acta Histochemica (2014),
down-regulated. Our present results were in accordance with pre-vious studies that have shown the placental E-cadherin expressionis raised in preeclampsia when compared with normal pregnancy(Li et al., 2003; Brown et al., 2005). Our results, together with

ARTICLE IN PRESSG ModelACTHIS-50843; No. of Pages 9
Y. Yu et al. / Acta Histochemica xxx (2014) xxx–xxx 7
Fig. 5. Knockdown of Notch-1 inhibited NF-�B pathway in JEG3 cells. Cells were transfected with shRNAs for 48 h, and then subjected to Western blot analysis to determinet -�B p� A cel
piE
NapoNautwi2ip
1wcho
R
A
A
he relative phosphorylation level of I�B� (A) and the nuclear translocation of NF-Actin and lamin A served as endogenous controls. ***P < 0.001 versus control shRN
revious reported studies suggest that Notch-1 may regulate thenvasion of placental trophoblasts by affecting E-cadherin-relatedMT process.
Wang et al. (2006) have demonstrated that down-regulation ofotch-1 inhibits invasion through inactivation of NF-�B in pancre-tic cancer cells (Wang et al., 2006), implying the NF-�B signalingathway involved Notch-1 induced cancer cell invasion. In supportf this earlier research, our results showed that the suppression ofotch-1 significantly reduced I�B� phosphorylation in JEG3 cellsnd subsequently inhibited the nuclear translocation of NF-�B sub-nit p65. We thus conclude that knockdown of Notch-1 may reducehe trophoblast cell invasion by suppressing NF-�B signaling path-ay. Moreover, because NF-�B pathway is also observed to be
nvolved in the regulation of E-cadherin expression (Zhang et al.,011) and MMP activity (Felx et al., 2006; Park et al., 2010) dur-
ng cell invasion, the corresponding regulatory mechanisms inreeclampsia need to be fully elucidated in the future.
In summary, we have demonstrated that knockdown of Notch- by shRNA suppresses the migration and invasion of JEG3 cells,hich is associated with decreased MMPs, inhibition of EMT pro-
ess and inactivation of NF-�B signaling pathway. Our researchas delineated an important role of Notch-1 in the pathogenesisf human preeclampsia in vitro.
eferences
nin SA, Vince G, Quenby S. Trophoblast invasion. Hum Fertil
Please cite this article in press as: Yu Y, et al. RNA interference-medown-regulates matrix metalloproteinases and suppresses NF-�B sighttp://dx.doi.org/10.1016/j.acthis.2014.03.003
(Camb) 2004;7:169–74.rtavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: cell
fate control and signal integration in development. Science1999;284:770–6.
65 (B). Data were presented as mean ± standard deviation (SD) (n = 3 per group).ls.
Bianchi S, Dotti MT, Federico A. Physiology and pathology of notchsignalling system. J Cell Physiol 2006;207:300–8.
Bischof P, Martelli M, Campana A, Itoh Y, Ogata Y, Nagase H.Importance of matrix metalloproteinases in human trophoblastinvasion. Early Pregnancy 1995;1:263–9.
Brown LM, Lacey HA, Baker PN, Crocker IP. E-cadherin in theassessment of aberrant placental cytotrophoblast turnover inpregnancies complicated by pre-eclampsia. Histochem Cell Biol2005;124:499–506.
Canel M, Serrels A, Frame MC, Brunton VG. E-cadherin–integrincrosstalk in cancer invasion and metastasis. J Cell Sci2013;126:393–401.
Cano A, Perez-Moreno MA, Rodrigo I, Locascio A, Blanco MJ,del Barrio MG, et al. The transcription factor Snail controlsepithelial-mesenchymal transitions by repressing E-cadherinexpression. Nat Cell Biol 2000;2:76–83.
Cobellis L, Mastrogiacomo A, Federico E, Schettino MT, De Falco M,Manente L, et al. Distribution of Notch protein members in nor-mal and preeclampsia-complicated placentas. Cell Tissue Res2007;330:527–34.
Felx M, Guyot MC, Isler M, Turcotte RE, Doyon J, KhatibAM, et al. Endothelin-1 (ET-1) promotes MMP-2 andMMP-9 induction involving the transcription factor NF-kappaB in human osteosarcoma. Clin Sci (Lond) 2006;110:645–54.
Ferretti C, Bruni L, Dangles-Marie V, Pecking AP, Bellet D. Molecularcircuits shared by placental and cancer cells, and their implica-tions in the proliferative, invasive and migratory capacities of
diated knockdown of Notch-1 inhibits migration and invasion,naling pathway in trophoblast cells. Acta Histochemica (2014),
trophoblasts. Hum Reprod Update 2007;13:121–41.Fisher SJ. The placental problem: linking abnormal cytotrophoblast
differentiation to the maternal symptoms of preeclampsia.Reprod Biol Endocrinol 2004;2:53.

ING ModelA
8 chemi
F
G
G
H
H
H
J
K
K
K
K
L
L
L
M
M
M
N
O
P
ARTICLECTHIS-50843; No. of Pages 9
Y. Yu et al. / Acta Histo
itzgerald JS, Poehlmann TG, Schleussner E, Markert UR. Tro-phoblast invasion: the role of intracellular cytokine signallingvia signal transducer and activator of transcription 3 (STAT3).Hum Reprod Update 2008;14:335–44.
ao W, Sweeney C, Connolly M, Kennedy A, Ng CT, McCormickJ, et al. Notch-1 mediates hypoxia-induced angiogen-esis in rheumatoid arthritis. Arthritis Rheum 2012;64:2104–13.
etsios S, Chen GT, MacCalman CD. Regulation of beta-cateninmRNA and protein levels in human villous cytotrophoblastsundergoing aggregation and fusion in vitro: correlation withE-cadherin expression. J Reprod Fertil 2000;119:59–68.
agemann T, Wilson J, Kulbe H, Li NF, Leinster DA, Charles K, et al.Macrophages induce invasiveness of epithelial cancer cells viaNF-kappa B and JNK. J Immunol 2005;175:1197–205.
annan NJ, Paiva P, Dimitriadis E, Salamonsen LA. Models for studyof human embryo implantation: choice of cell lines? Biol Reprod2010;82:235–45.
ansson SR, Gram M, Akerstrom B. Fetal hemoglobin in preeclamp-sia: a new causative factor, a tool for prediction/diagnosisand a potential target for therapy. Curr Opin Obstet Gynecol2013;25:448–55.
i L, Brkic J, Liu M, Fu G, Peng C, Wang YL. Placental trophoblastcell differentiation: physiological regulation and pathologi-cal relevance to preeclampsia. Mol Aspects Med 2013;34:981–1023.
anasaki K, Kalluri R. The biology of preeclampsia. Kidney Int2009;76:831–7.
okkinos MI, Murthi P, Wafai R, Thompson EW, Newgreen DF.Cadherins in the human placenta—epithelial-mesenchymaltransition (EMT) and placental development. Placenta2010;31:747–55.
ovats S, Main EK, Librach C, Stubblebine M, Fisher SJ, DeMars R. Aclass I antigen, HLA-G, expressed in human trophoblasts. Science1990;248:220–3.
rausch M, Kroepil F, Lehwald N, Lachenmayer A, Schott M,Anlauf M, et al. Notch 1 tumor expression is lacking in highlyproliferative pancreatic neuroendocrine tumors. Endocrine2013;44:182–6.
ain KY, Roberts JM. Contemporary concepts of the patho-genesis and management of preeclampsia. JAMA 2002;287:3183–6.
i HW, Cheung AN, Tsao SW, Cheung AL, WS O. Expression of E-cadherin and beta-catenin in trophoblastic tissue in normal andpathological pregnancies. Int J Gynecol Pathol 2003;22:63–70.
i JK, Xiong Q, Zhou S, Yang PF. Differences between the expressionof matrix metalloproteinase-2, 9 in preeclampsia and nor-mal placental tissues. Zhonghua Fu Chan Ke Za Zhi 2007;42:73–5.
andl M, Ghaffari-Tabrizi N, Haas J, Nohammer G, Desoye G.Differential glucocorticoid effects on proliferation and inva-sion of human trophoblast cell lines. Reproduction 2006;132:159–67.
cMaster MT, Zhou Y, Fisher SJ. Abnormal placentation and thesyndrome of preeclampsia. Semin Nephrol 2004;24:540–7.
uller PY, Janovjak H, Miserez AR, Dobbie Z. Processing of geneexpression data generated by quantitative real-time RT-PCR.Biotechniques 2002;32:1372–4, 76, 78–79.
agase H, Visse R, Murphy G. Structure and function of matrix met-alloproteinases and TIMPs. Cardiovasc Res 2006;69:562–73.
nai Y, Suzuki J, Maejima Y, Haraguchi G, Muto S, Itai A, et al. Inhi-bition of NF-{kappa}B improves left ventricular remodeling and
Please cite this article in press as: Yu Y, et al. RNA interference-medown-regulates matrix metalloproteinases and suppresses NF-�B sighttp://dx.doi.org/10.1016/j.acthis.2014.03.003
cardiac dysfunction after myocardial infarction. Am J PhysiolHeart Circ Physiol 2007;292:H530–8.
ark JH, Jeong YJ, Park KK, Cho HJ, Chung IK, Min KS, et al. Melit-tin suppresses PMA-induced tumor cell invasion by inhibiting
PRESSca xxx (2014) xxx–xxx
NF-kappaB and AP-1-dependent MMP-9 expression. Mol Cell2010;29:209–15.
Parr C, Watkins G, Jiang WG. The possible correlation of Notch-1and Notch-2 with clinical outcome and tumour clinicopatho-logical parameters in human breast cancer. Int J Mol Med2004;14:779–86.
Redman CW, Sargent IL. Latest advances in understandingpreeclampsia. Science 2005;308:1592–4.
Sahlgren C, Gustafsson MV, Jin S, Poellinger L, Lendahl U. Notchsignaling mediates hypoxia-induced tumor cell migration andinvasion. Proc Natl Acad Sci U S A 2008;105:6392–7.
Song LL, Peng Y, Yun J, Rizzo P, Chaturvedi V, Weijzen S, et al. Notch-1 associates with IKKalpha and regulates IKK activity in cervicalcancer cells. Oncogene 2008;27:5833–44.
Tian X, Liu Z, Niu B, Zhang J, Tan TK, Lee SR, et al. E-cadherin/beta-catenin complex and the epithelial barrier. J Biomed Biotechnol2011;2011:567305.
Toth M, Sohail A, Fridman R. Assessment of gelatinases (MMP-2 and MMP-9) by gelatin zymography. Methods Mol Biol2012;878:121–35.
Tripathi V, Popescu NC, Zimonjic DB. DLC1 induces expression ofE-cadherin in prostate cancer cells through Rho pathway andsuppresses invasion. Oncogene 2013.
Vicovac L, Aplin JD. Epithelial-mesenchymal transition during tro-phoblast differentiation. Acta Anat (Basel) 1996;156:202–16.
Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitorsof metalloproteinases: structure, function, and biochemistry.Circ Res 2003;92:827–39.
Wang Z, Banerjee S, Li Y, Rahman KM, Zhang Y, Sarkar FH.Down-regulation of notch-1 inhibits invasion by inactivation ofnuclear factor-kappaB, vascular endothelial growth factor, andmatrix metalloproteinase-9 in pancreatic cancer cells. CancerRes 2006;66:2778–84.
Wang Z, Li Y, Banerjee S, Kong D, Ahmad A, Nogueira V, et al. Down-regulation of Notch-1 and Jagged-1 inhibits prostate cancer cellgrowth, migration and invasion, and induces apoptosis via inac-tivation of Akt, mTOR, and NF-kappaB signaling pathways. J CellBiochem 2010a;109:726–36.
Wang Z, Li Y, Sarkar FH. Notch signaling proteins: legitimatetargets for cancer therapy. Curr Protein Pept Sci 2010b;11:398–408.
Winn VD, Gormley M, Fisher SJ. The impact of preeclampsia on geneexpression at the maternal–fetal interface. Pregnancy Hyper-tens 2011;1:100–8.
Winn VD, Gormley M, Paquet AC, Kjaer-Sorensen K, KramerA, Rumer KK, et al. Severe preeclampsia-related changes ingene expression at the maternal–fetal interface include sialicacid-binding immunoglobulin-like lectin-6 and pappalysin-2.Endocrinology 2009;150:452–62.
Wu Y, Deng J, Rychahou PG, Qiu S, Evers BM, Zhou BP. Stabilizationof Snail by NF-kappaB is required for inflammation-induced cellmigration and invasion. Cancer Cell 2009;15:416–28.
Xiao LJ, Lin P, Lin F, Liu X, Qin W, Zou HF, et al. ADAM17targets MMP-2 and MMP-9 via EGFR-MEK-ERK pathway acti-vation to promote prostate cancer cell invasion. Int J Oncol2012;40:1714–24.
Zhang K, Zhaos J, Liu X, Yan B, Chen D, Gao Y, et al. Activation ofNF-B upregulates Snail and consequent repression of E-cadherinin cholangiocarcinoma cell invasion. Hepatogastroenterology2011;58:1–7.
Zhang Y, Li B, Ji ZZ, Zheng PS. Notch1 regulates the growth of humancolon cancers. Cancer 2010;116:5207–18.
Zhang Z, Zhang L, Jia L, Cui S, Shi Y, Chang A, et al. AP-2alpha
diated knockdown of Notch-1 inhibits migration and invasion,naling pathway in trophoblast cells. Acta Histochemica (2014),
suppresses invasion in BeWo cells by repression of matrixmetalloproteinase-2 and -9 and up-regulation of E-cadherin.Mol Cell Biochem 2013;381:31–9.

ING ModelA
chemi
Z
Z
ARTICLECTHIS-50843; No. of Pages 9
Y. Yu et al. / Acta Histo
hao H, Yang Z, Wang X, Zhang X, Wang M, Wang Y, et al. Triptolideinhibits ovarian cancer cell invasion by repression of matrix
Please cite this article in press as: Yu Y, et al. RNA interference-medown-regulates matrix metalloproteinases and suppresses NF-�B sighttp://dx.doi.org/10.1016/j.acthis.2014.03.003
metalloproteinase 7 and 19 and upregulation of E-cadherin. ExpMol Med 2012;44:633–41.
hou Y, McMaster M, Woo K, Janatpour M, Perry J, Karpanen T,et al. Vascular endothelial growth factor ligands and receptors
PRESSca xxx (2014) xxx–xxx 9
that regulate human cytotrophoblast survival are dysregulatedin severe preeclampsia and hemolysis, elevated liver enzymes,
diated knockdown of Notch-1 inhibits migration and invasion,naling pathway in trophoblast cells. Acta Histochemica (2014),
and low platelets syndrome. Am J Pathol 2002;160:1405–23.Zhu JY, Pang ZJ, Yu YH. Regulation of trophoblast invasion:
the role of matrix metalloproteinases. Rev Obstet Gynecol2012;5:e137–43.