Physics’401:’ Quantum’Mechanics’I Chapter’4alrudolph/classes/phy402/PDFs/E5_Chapter 4...
Transcript of Physics’401:’ Quantum’Mechanics’I Chapter’4alrudolph/classes/phy402/PDFs/E5_Chapter 4...
The ground state energy of the particle in
a 3D box is
What is the energy of the 1st excited state?
• A) 4ε B) 5ε C) 6ε D) 8ε E) 9ε
12 +12 +12( ) π
22
2ma2 = 3ε .
83
In the 3D infinite square well,what is the degeneracy of the energy
corresponding to the state(nx , ny , nz ) = (1, 2, 3)?
A) 1 B) 3 C) 4 D) 6 E) 9
84
In Cartesian coordinates, the volume element is dx dy dz. In spherical coordinates, the volume element is
A) r2sinθ cosφ dr dθ dφB) sinθ cosφ dr dθ dφC) r2cosθ sinφ dr dθ dφD) r sinθ cosφ dr dθ dφE) r2sinθ dr dθ dφ
99
A planet is in elliptical orbit about the sun.
The torque, on the planet about the sun is:
A) Zero alwaysB) Non-zero alwaysC) Zero at some points, non-zero at
others.95
€
τ = r × F
P A
The magnitude of the angular momentum of the planet about the sun is:
A) Greatest at the perihelion point, PB) Greatest at the aphelion point, AC) Constant everywhere in the orbit
96
€
L = r × p
P A
In Cartesian coordinates, thenormalization condition is
In spherical coordinates, the normalization integral has limits of integration:
E) None of these
€
dx−∞
∞
∫ dy−∞
∞
∫ dz−∞
∞
∫ Ψ2
=1.
100
€
A) dr0
+∞
∫ dθ0
2π
∫ dϕ0
π
∫ …
€
B) dr−∞
+∞
∫ dθ0
2π
∫ dϕ0
π
∫ …
€
C) dr0
+∞
∫ dθ0
2π
∫ dϕ0
2π
∫ …
€
D) dr−∞
+∞
∫ dθ0
π
∫ dϕ0
π
∫ …
A and B are positive constants. r is radial distance (0 ≤ r < ∞). Using the whiteboards:
Sketch and
What does the graph look like?
rA−
111
€
Br2
€
y(r) =Br2−Ar
Consider He+ (1 e- around a nucleus, Q= 2e). If you look at “Balmer lines” (e- falling from higher n down to n=2) what part of the spectrum do you expect the emitted radiation to fall in?
€
En =E1
n2 , with E1 = −(ke2)2me /22
A)VisibleB) IRC) UVD) It’s complicated, not obvious at all.
Recall, for hydrogen:
The spectrum of "Perkonium" has only 3 emission lines.
114
wavelength(nm)200 300 400 500 600
5 eV 3 eV 2 eV
Which energy level structure is consistent with the spectrum?
E(eV)
–2–3
–5
–2
–4–5
–7 –7
–5
–10
–5
–7–8
(A) (B) (C) (D)
As indicated in the figure, the n = 2, l = 0 state and the n = 2, l = 1 state happen to have the
same energy (given by E2 = E1/22 ).Do these states have the same radial
wavefunction R(r) ?
x
Veff
l = 0
l = 1 l = 2
n = 1
n = 2n = 3
4
A) Yes B) No
120
Consider an electron in the ground state of an H-atom. The wavefunction is
0(r) Aexp( r / a )ψ = −Where is the electron more likely to be found?
A)Within dr of the origin (r = 0)
B) Within dr of a distance r = a0 from the origin?
A
B
x
y
r = a0
Are you here today?
A. YesB. NoC. Eigenfunction, eigenvalue, eigenfunction, eigenvalue… what’s the difference again?
We are solving the equation
What, then, is the full 3-D wave function for hydrogen atom stationary states?A) u(r,θ,φ) B) u(r)Ylm(θ,φ)
C) ru(r)Ylm(θ,φ) D) r2u(r)Ylm(θ,φ)
E) None of these
−2
2md 2udr2
+−ke2
r+2l(l +1)2mr2
"
#$
%
&'u = Eu
True (A) or False (B)
Any arbitrary stationary state of an electron bound in the H-atom potential can always be written as
with suitable choice of n, l, and m.
ψ n,l ,m (r,θ ,φ) = Rnl (r)Ylm (θ ,φ),
Suppose at t=0,
Is Ψ (r,t) given very simply byΨ (r,t =0) e-iEt/ħ?
A)Yes, that’s the simple resultB) No, it’s more complicated (a superposition of two states with different t dependence => “sloshing”)
€
Ψ(r,t = 0) =12(ψ210 +ψ200)
Suppose at t=0,
€
Ψ(r,t = 0) =12(ψ 200 +ψ 300)
Is Ψ (r,t) given very simply byΨ (r,t =0) e-iEt/ħ?
A)Yes, that’s the simple resultB) No, it’s more complicated (a superposition of two states with different t dependence => “sloshing”)
How many of the following transitions to the 2p in an H-atom will result in emission of a photon ?
s p d f
n = 1
2
3
4
E
A) all of them: 11 B) None of them: 0 C) 8
D) 9 E) 6
0
1
2
3
4
5
6
7
8
9
10
11
12
13
0-10 10-20 20-30 30-40 40-50 50-60 60-70 70-80 80-90 90-100+
Fre
qu
en
cy
Score
P402 W16 Exam Distribution
Midterm Final
Midterm results
Average = 70
The angular momentum operator is
with e.g.
Is Hermitian? (Hint: Is Lz Hermitian?)
A) Yes B) NoC) Only Lz is (Lx and Ly are not) D) Lz is not (but Lx and Ly are) E) Are you joking here? Can I do this as a
clicker question?
€
ˆ L x = ˆ y p z − ˆ z p y, ..., ˆ L z = ˆ x p y − ˆ y p x
€
ˆ L = ˆ r × ˆ p = ( ˆ L x, ˆ L y, ˆ L z )
€
ˆ L
The commutator,
zero or non-zero?
A) ZeroB) Non-zeroC) Sometimes zero, sometimes non-zero
€
ˆ y p z, ˆ x p z[ ]
97
The commutator,
zero or non-zero?
A) ZeroB) Non-zeroC) Sometimes zero, sometimes non-zero
€
Lz2, ˆ L z[ ]
98
Postulates of Quantum Mechanics(1) The state of a particle is completely represented by a normalized vector in Hilbert space, which we call |ψ>
(2) All physical observables, Q, are associated with Hermitian operators Q, and the expectation value of Q in some state |ψ> is <ψ|Q|ψ>(3) A measurement of Q on a particle in state |ψ> is certain to return a particular value, λ , iff ("if and only if") Q|ψ> = λ|ψ>, (i.e. if and only if |ψ> is already an eigenvector of Q, with eigenvalue λ)
(3a) If you measure Q in any state |ψ>, you are certain to obtain one of the eigenvalues of Q. The probability of measuring some eigenvalue λ is given by |<uλ|ψ>|2, where |uλ> is defined to be the eigenvector of Q with eigenvalue λ
(3b) After a measurement gives you the value λ, the system will collapse into the state |uλ>
(4) The time evolution of the state |ψ> is given by Schrodinger's equation:
i∂ψ∂t
= H ψ
SJP QM 3220 3D 1
Page H-32 M. Dubson, (typeset by J. Anderson) Mods by S. Pollock Fall 2008
(ThispagefromQMnotesofProf.RogerTobin,PhysicsDept,TuftsU.)
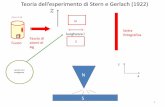
Stern-Gerlach Experiment (W. Gerlach & O. Stern, Z. Physik 9, 349-252 (1922).
€
F = ∇ µ • B ( ) = (
µ • ∇ ) B (in current free regions), or here,
F = ˆ z (µ
z
∂Bz
∂z)(thisisalittle
crude‐seeGriffithsExample4.4forabettertreatment,butthisgivesthemainidea)
Deflectionofatomsinz‐directionisproportionaltoz‐componentofmagnetic
momentµz,whichinturnisproportionaltoLz.Thefactthattherearetwobeamsis
proofthatl=s=½.Thetwobeamscorrespondtom=+1/2andm=–1/2.Ifl=1,
thentherewouldbethreebeams,correspondingtom=–1,0,1.Theseparationof
thebeamsisadirectmeasureofµz,whichprovidesproofthat
Theextrafactorof2intheexpressionforthemagneticmomentoftheelectronis
oftencalledthe"g‐factor"andthemagneticmomentisoftenwrittenas
.Asmentionedbefore,thiscannotbededucedfromnon‐relativistic
QM;itisknownfromexperimentandisinserted"byhand"intothetheory.



































































![[Topic 2-Endogeneity] 1/33 Topics in Microeconometrics William Greene Department of Economics Stern School of Business.](https://static.fdocument.org/doc/165x107/5697bf731a28abf838c7f4dd/topic-2-endogeneity-133-topics-in-microeconometrics-william-greene-department.jpg)