Interleukin-18 Production Following Murine Cardiac Transplantation: Correlation with Histologic...
Transcript of Interleukin-18 Production Following Murine Cardiac Transplantation: Correlation with Histologic...

JOURNAL OF INTERFERON AND CYTOKINE RESEARCH 21:1–9 (2001)Mary Ann Liebert, Inc.
Interleukin-18 Production Following Murine CardiacTransplantation: Correlation with Histologic Rejection
and the Induction of IFN-g
DAVID G. AFFLECK,1 DAVID A. BULL,2 AIDA ALBANIL,2 YUANLIN SHAO,1 JOHN BRADY,2
SHREEKANTH V. KARWANDE,2 ERNST J. EICHWALD,3 and JANE SHELBY1
ABSTRACT
Interleukin-18 (IL-18) and IL-12 have been shown to play an important role in the induction of interferon-c(IFN-c ). IFN-c induces the proliferation of T cells and natural killer (NK) cells and augments the Th1 im-mune cascade. The role of IL-18 and IL-12 in the induction of IFN-c following allogeneic heart transplanta-tion has not been described. We sought to characterize the IL-12 and IL-18 response to murine allogeneicheart transplantation, particularly with respect to IFN-c production and histologic transplant rejection. Forty-eight heterotopic heart transplants were performed in two groups of mice: syngeneic C3H/HeN to C3H/HeNmice and allogeneic BALB/C to C3H/HeN mice. Transplants were followed out to 2, 6, 10, and 14 days. Sixtransplants were performed in each group. Serum and splenic samples were used to evaluate the cytokine re-sponse by ELISA. Explanted heart tissue was processed for evidence of histologic rejection, and RT-PCR wasperformed to evaluate the IL-12, IL-18, and IFN-c signal qualitatively. Analysis of variance (ANOVA), Fisher’sprojected least significant difference (PLSD) was used for statistical analysis. Transplant rejection occurredin the allogeneic group histologically by day 6 and clinically by day 10. Serum IFN-c levels rose significantlyby day 6 in the allogeneic group and then continued to rise in the splenocyte cultures. Serum IL-18 also rosesignificantly in the allogeneic group at day 6 compared with syngeneic group. RT-PCR revealed that the al-logeneic tissue contained an increased signal for IL-12, IL-18, and IFN-c beginning at day 6 and peaking atday 10 after transplant. Beginning 6 days after transplantation, IL-12 and IL-18 appear to play a significantrole in the induction of IFN-c in allogeneic heart transplants.
1
INTRODUCTION
CLINICAL HEART TRANSPLANTATION has become a viable ther-apeutic option for patients with end-stage cardiac failure.
A major obstacle to the long-term durability of cardiac trans-plants remains the host response to allogeneic transplantation.An improved understanding of the posttransplant immune cas-cade of T cells, B cells, natural killer (NK) cells, and cytokinerelease may improve the management of transplant rejectionand improve long-term graft survival.(1,2)
Interferon-g (IFN-g) plays a central role in the posttransplantimmune response.(1–3) IFN-g induces the proliferation of Tcells, potentiates the NK response, and promotes the Th1 im-
mune cascade.(4–6) These responses enhance the recipient re-sponse to foreign donor tissue. Several secreted cytokines, in-cluding interleukin-2 (IL-2), IL-12, and IL-18, may play aprominent role in regulating the IFN-g response to foreign tis-sue.(7,8) Recent studies suggest that IL-12 and IL-18 may actsynergistically to increase IFN-g levels.(9–12)
Although IL-18 has been shown to play a significant role inthe cellular and humoral response to stress, inflammation andinfection,(13–16) its role in the cytokine response following hearttransplantation is unknown. The purpose of this study is to char-acterize the IL-12 and IL-18 responses following allogeneicheart transplantation, particularly in relation to IFN-g produc-tion and transplant rejection.
1Department of Surgery, 2Division of Cardiothoracic Surgery, and 3Department of Pathology, University of Utah Health Sciences Center,School of Medicine 3C127, Salt Lake City, UT 84132.

MATERIALS AND METHODS
Transplant model
Heterotopic heart transplants were performed in C3H/HeNmice after the model of Corry et al.,(17) as approved by the In-stitutional Animal Care and Use Committee at the Universityof Utah. Allogeneic transplants were performed between donorBALB/C mice and C3H/HeN mice. Syngeneic transplants wereperformed between C3H/HeN and C3H/HeN mice. The trans-plants were performed by primary vascularization of the donororgan to the recipient abdominal aorta and vena cava. Animalswere recovered and monitored daily for graft function by gen-tle palpation of the abdominal graft.
Transplant groups
Syngeneic and allogeneic transplants were performed in 48mice. Syngeneic transplants were performed in 24 mice andharvested at 2, 6, 10, and 14 days after transplantation, with 6transplants in each group. Similarly, allogeneic transplants wereperformed and harvested 2, 6, 10, and 14 days after transplan-tation, with 6 transplants in each group. Unoperated C3H/HeNmice served as controls for serum and splenocyte cytokine mea-surements. Heart tissue for PCR controls was obtained from un-touched BALB/C hearts. Differences in serum and splenocytecytokine levels were compared between transplant groups us-ing ANOVA, Fisher’s PLSD (Statview) (Abacus Concepts,Berkeley, CA).
Tissue preparation
Transplanted hearts, serum, and spleens were taken fromeach donor on the day of killing. Transplanted hearts were sec-tioned through the ventricles, with one section preserved forhistologic processing. Each section from the transplanted heartwas fixed in formaldehyde and sectioned in paraffin. Sectionswere counterstained with hematoxylin and eosin (H&E). Serumsamples and splenocytes were processed for cytokine ELISA.
Serum cytokine ELISA
Serum IL-12 ELISA was performed using primary and sec-ondary antibodies to the p40 subunit of IL-12 (PharMingen, SanDiego, CA). The optical reading was performed in triplicate andcompared to a standard. The lower limit of detection was 15ng/ml.
Serum IL-18 ELISA was performed using a mouse IL-18 as-say (Medical & Biological Laboratories, Nagoya, Japan) em-ploying two monoclonal antibodies (mAb) to two different mouseIL-18 epitopes. The lower limit of detection was 30 pg/ml.
Serum IFN-g ELISA was performed using primary and sec-ondary antibodies (PharMingen), read in triplicate, and mea-sured against a standard curve. The lower limit of detection was15 ng/ml.
Splenocyte cultures/ELISA
Splenocytes from each mouse were harvested at death andcultured in RPMI (Gibco-BRL, Gaithersburg, MD) plus Nu-tridoma (Boehringer Mannheim, Indianapolis, IN) for 24 h at5% CO2 at a cell density of 1 3 107/ml. Activation of the
splenocytes was achieved using a-CD3 antibody (1 mg/ml) tothe T cell receptor (PharMingen). Supernatant from the culturedwells was processed in a similar fashion to the serum cytokineassays. All samples were read in triplicate against a standardcurve.
RT-PCR: Analysis of graft cytokine signal
Tissue from each transplanted heart was used to evaluateeach cytokine RNA signal arising from the graft. Control PCRtissue was obtained from untouched BALB/c mice. Total RNAwas extracted from 30–40 mg of explanted heart tissue usingthe SV Total RNA System (Promega, Madison WI). IsolatedRNA was assessed for purity using spectrophotometric ab-sorbance at 260–280 nm using a spectrometer from Shimadzu(Columbia, MD). OD readings were between 1.9 and 2.
Isolated RNA (1 mg) from each sample was reverse tran-scribed using the Promega Reverse Transcription System.cDNA generated from reverse transcription was amplified us-ing PCR at 25, 30, and 35 cycles. Thirty cycles provided opti-mum saturation curves based on internal standards.
PCR controls were pBR322 Vector DNA and b-actin(Promega). The pBR322 Vector DNA was used as an internalpositive PCR control. The vector contains restriction sites(clockwise) for BamH1 (ATG CGT CCG GCG TAG A) andEcoR1 (GTA TCA CGA GGC CCT T), which allow amplifi-cation of the plasmid and produce a reliable band and verifythe integrity of the PCR cycle. These primers were obtainedfrom Sigma (St Louis, MO). b-Actin primers were used as in-ternal controls to ensure equivalent specimen processing acrosssamples. The actin primer sequences used were 59-TCA TGAAGT GTG ACG TTG ACA TCC GT-39 sense and 59-CCTAGA AGC ATT TGC GGT GCA CGA TG-39 antisense.
Negative PCR controls were performed using each specificprimer pair, 1 mg of isolated cDNA, without substrate for thePCR reaction. All negative controls were blank (data notshown).
PCR for IFN-g was based on the primer pairs, 59-ACA GTCATT GAA AGC CTA GA-3 sense and 59-TGT GAT TCA ATGACG CTT AT-3 antisense. The IL-12 PCR was based on thep40 subunit of the IL-12 transcript. The primers used were 59-AGA TGA CAT CAC CTG GAC CT-39 sense and 59-GCCATG AGC ACG TGA ACC GT-39 antisense. IL-18 primersused were 59-GGC CCA GGA ACA ATG GCT GCC-39 senseand 59-GGG TCA CAG CCA GTC CTC TTA C-39 antisense.All primers were synthesized from GIBCO-BRL.
PCR products were stained with ethidium bromide and runon 2% agarose gels. The resulting gels were imaged using theStratagene Eagle Eye II system (La Jolla, CA). Band intensitywas compared between groups qualitatively. Band intensity wasfurther compared using Scion Image Digital Gel Analysis Soft-ware (Scion Corporation, Frederick, MD).
RESULTS
The allogeneic and syngeneic transplant groups differed withrespect to serum and splenocyte cytokine profiles, transplantheart tissue PCR cytokine signal, histologic rejection, and graftfunction.
AFFLECK ET AL.2

IL-18 PRODUCTION FOLLOWING TRANSPLANTATION 3
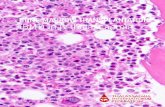
FIG. 1. Histologic sections from heterotopic murine cardiac transplants. (A) Day 6 syngeneic transplant. Normal myocardialarchitecture can be appreciated. There is evidence of mild interstital changes that presumably represent injury resulting from hy-poxia or mechanical trauma at the time of transplantation. (B) Day 6 allogeneic transplant. Allogeneic transplants show evidenceof significant interstitial change compared with the corresponding syngeneic day 6 hearts. Myocyte nuclear changes can be ap-preciated as well as increasing fibroblastic infiltration. (C) Day 10 syngeneic transplant. Myocytes appear healthy, with minimalinterstitial changes compared with (D) day 10 allogeneic transplant. By day 10, the allogeneic hearts demonstrate loss of normalmyocyte architecture with interstitial hemorrhage, increasing fibroblast infiltration, and vascular thrombosis. (E) Day 14 syn-geneic transplant. Myocytes appear healthy, with minimal interstitial change. There is no evidence of significant scar or vascu-lar change. Day 14 syngeneic transplants continue to beat spontaneously at the time of explanation. (F) Day 14 allogeneic trans-plant. By day 14, the allogeneic tissue shows extensive interstitial fibrotic replacement of myocytes, vascular thrombosis, andhemorrhage.
A B
C D
E F

AFFLECK ET AL.4
FIG. 2. (A) Serum IL-12 levels are not significantly different in the syngeneic (SYN) and allogeneic (ALLO) groups at day 2(D2) or day 6 (D6). By day 10 (D10), serum IL-12 levels are significantly higher in the ALLO group compared with the SYNgroup (2.42 6 0.05 ng/ml ALLO vs. 1.95 6 0.05 ng/ml SYN, p 5 0.01). Further, there is a significant rise from D6 to D10 amongthe ALLO group (p , 0.0001). A similar rise from D6 to D10 is not seen among the SYN transplants. (B) IL-18 levels in thecontrol mice were 171.3 6 10.2 pg/ml. Compared with controls, IL-18 levels fall in the SYN mice (p 5 0.004) and the ALLOmice (p 5 0.0002) 2 days after transplantation (D2). By D6, the ALLO transplants show a significant rise in IL-18 (90.5 6 5.7pg/ml ALLO D2 vs. 160.9 6 20.4 pg/ml ALLO D6, p 5 0.0005). A similar rise from D2 to D6 is not seen in the SYN group(p 5 0.12). Further, there is a significant drop in IL-18 among the SYN group by D10 and D14. (C) Serum IFN-g levels. Base-line IFN-g levels are 0.8 6 0.4 ng/ml. Similar to the IL-18 data, the levels drop in both the SYN and ALLO groups at D2. FromD2 to D6 the ALLO IFN-g level rises significantly (,0.03 ng/ml ALLO D2 vs. 2.5 6 1.0 ng/ml ALLO D6, p 5 0.0001). TheD2 to D6 rise in not significant (p 5 0.9). Further, the difference between the SYN D6 and ALLO D6 is significant (p 5 0.01).Both the SYN and ALLO serum IFN-g levels fall significantly on D10 and D14. This fall occurs in spite of the clinical and his-tologic findings that the ALLO grafts are necrotic, thrombosed, nonfunctional, and clearly rejected.

Histologic rejection/graft function
Syngeneic transplants functioned throughout the course ofthe study. At each time point, days 2, 6, 10, and 14, each heartappeared viable at the time of explantation and continued tobeat vigorously. Histologic examination revealed only a mild
injury pattern, consistent with ischemia at the time of transplantas well as minimal mechanical trauma (Fig. 1). Histologically,the syngeneic hearts had healthy appearing myocytes, minimalfibrosis, and intact architecture of capillaries and interstitiumthrough 14 days after transplantation.
In contrast, among allogeneic transplants, by day 6, 4 of 6
IL-18 PRODUCTION FOLLOWING TRANSPLANTATION 5
FIG. 3. (A) Splenocyte IL-12 levels. There is induction of IL-12 among the allogeneic (ALLO) transplants. The IL-12 levelsare higher on day 6 (D6) (1.21 6 0.5 ng/ml syngeneic [SYN] D6 vs. 2.7 6 0.4 ng/ml ALLO D6, p 5 0.001) and D10 (1.49 60.2 ng/ml SYN D10 vs. 2.72 6 0.4 ng/ml ALLO D10, p 5 0.01). (B) Splenocyte IL-18 levels. Both SYN and ALLO transplantgroups are significantly higher than control levels at each time point. Nevertheless, there was no significant difference betweenthe SYN and ALLO groups at any of the time points. (C) Splenocyte IFN-g levels. A difference in splenocyte IFN-g levels be-tween the SYN and ALLO groups is demonstrated on D10 and D14. Although the D10 ALLO level is higher than the D10 SYNlevel, this did not reach statistical significance (p 5 0.4). By D14, the difference is accentuated by falling SYN IFN-g levels andis substantially higher among the ALLO transplants (31.4 6 6.8 ng/ml SYN D14 vs. 52.3 6 8.4 ng/ml ALLO D14, p 5 0.01).

transplants no longer demonstrated spontaneous contraction,and by day 10, all allogeneic transplants appeared hemorrhagic,necrotic, thrombosed, and without any evidence of contraction.Histologically, these allogeneic transplants demonstrated in-creasing lymphocytic infiltration, interstitial fibrotic replace-ment of myocytes, capillary leak reflected by diffuse intersti-tial red blood cell extravasation, thrombosis of capillaries, andmyocyte morphology changes. In this setting, the serum cy-tokine data must be interpreted with the understanding that theallogeneic transplants thrombosed completely by day 10, andthus, the macrophages and antigen-presenting cells (APC) thatinfiltrated the graft did not access the general circulation.
Serum cytokine measurements
IL-12. Figure 2 demonstrates the serum profile of IL-12 inboth the syngeneic and allogeneic transplants. Of note, serumIL-12 levels in both allogeneic and syngeneic transplants aremoderately low at both 2 and 6 days posttransplant. There wasno difference in the IL-12 levels between these two groups atdays 2 and 6. At day 10, the serum IL-12 level is higher in theallogeneic than in the syngeneic transplants (2.42 6 0.05 ng/mlvs. 1.95 6 0.05 ng/ml, p 5 0.01). This elevation in serum IL-12 persisted through day 14 in the allogeneic transplants. Fur-ther, there is a significant incremental rise in IL-12 among theallogeneic transplants from day 6 to day 10 (p , 0.0001). Asimilar rise is not seen in the syngeneic group from day 6 today 10. In conclusion, serum IL-12 levels increase in both syn-geneic and allogeneic groups, but the rise in IL-12 levels ismore pronounced in the allogeneic group.
IL-18. Serum IL-18 levels after transplantation are more dif-ficult to interpret. Baseline control IL-18 from untouched miceis 171.3 6 10.2 pg/ml. Two days after transplant, both the syn-geneic and allogeneic groups exhibited a significant drop in IL-18 levels compared with controls (p 5 0.004 and p 5 0.0002,respectively). Compared with day 2, allogeneic transplants atday 6 experienced a significant rise in IL-18 levels (90.5 6 5.7pg/ml vs. 160.9 6 20.4 pg/ml, p 5 0.0005). A similar rise wasnot seen in the syngeneic transplant group. Further, there wasa significant drop in serum IL-18 among the syngeneic groupby day 10 and day 14.
IFN-g. In the control mice, baseline IFN-g levels were0.8 6 0.4 ng/ml. Again, as in the case of IL-18, IFN-g lev-els at day 2 drop in both syngeneic and allogeneic groups.This is followed by a rise in the IFN-g levels in the allo-geneic group from , 0.03 ng/ml on day 2 to 2.5 6 1.0 ng/mlon day 6 (p 5 0.0001). A similar rise in IFN-g levels was notseen in the syngeneic group from day 2 to day 6. The dif-ference in the IFN-g levels between the syngeneic and allo-geneic groups at day 6 is significant (p 5 0.01). By day 10,both the syngeneic and allogeneic IFN-g serum levels dropsignificantly. This drop occurs in the setting of clinical andhistologic rejection.
Splenocyte a-CD3 cytokine measurements
IL-12. Splenocyte IL-12 data support the serum findings thatthere is a preferential IL-12 induction among the allogeneictransplants (Fig. 3A). There is a difference in IL-12 levels bothat day 6 (1.21 6 0.5 ng/ml syngeneic day 6 vs. 2.7 6 0.4 ng/ml
allogeneic day 6, p 5 0.001) and day 10 (1.49 6 0.2 ng/ml syn-geneic day 10 vs. 2.72 6 0.4 ng/ml allogeneic day 10, p 5
0.01).IL-18. Splenocyte IL-18 data reflected a generalized activa-
tion in all groups (Fig. 3B). Whereas all groups and time pointswere statistically higher than controls, there were no differencesbetween syngeneic and allogeneic groups.
IFN-g. Splenocyte production of IFN-g reflected an inter-esting pattern (Fig. 3C). Consistent with the serum data, allo-geneic transplants had higher IFN-g levels, although this wasnot evident until later in the posttransplant course. With theserum IFN-g data, the greatest difference between the syngeneicand allogeneic groups occurred on day 6. From the splenocytes,the difference in IFN-g levels is not demonstrable until day 14(31.4 6 6.8 ng/ml syngeneic day 14 vs. 52.3 6 8.4 ng/ml allo-geneic day 14, p 5 0.01).
AFFLECK ET AL.6
FIG. 4. (A) Agarose gel (2%) stained with ethidium bromide.RNA (1 mg) was isolated from each explanted heart, and IL-12mRNA was amplified using reverse transcription (RT) (cDNA)and PCR. The IL-12 signal was amplified using primers spe-cific for the p40 subunit of the protein. A representative lane isdepicted from each transplant group and time point. The IL-12signal is absent from the syngeneic (SYN) transplants. The al-logeneic (ALLO) group has a somewhat increased signal by day6, with a significant increase on day 10. This correlates withthe serum and splenocyte ELISA findings. Lane 1 is a size ref-erence ladder, which does not undergo PCR. Negative PCR con-trols (not shown) were blank. (B) The histogram displays therelative intensity of the PCR signal. Lane 1, Hyperladder sizereference; lane 2, control (untouched BALB/c heart tissue); lane3, ALLO day 2; lane 4, SYN, day 2; lane 5, ALLO day 6; lane6, SYN day 6; lane 7, ALLO day 10; lane 8, SYN day 10; lane9, ALLO day 14; lane 10, SYN day 14.

IL-12 PCR pattern
Analysis of the tissue IL-12 mRNA signal demonstrated atrend toward higher IL-12 signal among allogeneic mice (Fig.4). The allogeneic group begins to show increased IL-12 sig-nal on day 6 with a significant peak on day 10. IL-12 signalwas nearly undetectable among the syngeneic transplants at alltime points.
IL-18 PCR pattern
The IL-18 signal from the heart tissue appears to mirrorthe serum and heart tissue IFN-g signal (Fig. 5). RT-PCR re-veals that the IL-18 signal in the control group (untouchedBALB/c heart tissue) is initially relatively low. Consistent
with the serum IL-18 levels, the IL-18 mRNA signal dropsbelow the control level in both the syngeneic and allogeneicgroups on day 2. By day 6, the syngeneic IL-18 signal nearlydisappears. It then gradually increases back toward controlintensity by day 14. Among the allogeneic transplants, thepicture is quite different. After the initial drop in signal in-tensity at day 2, the IL-18 mRNA signal is increased 2–3-fold on days 6 and 10. By day 14, the allogeneic IL-18 mRNAsignal declines yet remains higher than that seen in the con-trol group.
IFN-g PCR pattern
The RT-PCR signal for IFN-g is quite distinct between thesyngeneic and allogeneic transplant groups (Fig. 6). Similar tothe serum findings, the initial PCR signal for IFN-g is absentat day 2. The syngeneic signal remains absent throughout all
IL-18 PRODUCTION FOLLOWING TRANSPLANTATION 7
FIG. 5. (A) Agarose gel (2%) stained with ethidium bromide.RNA (1 mg) was isolated from each explanted heart, and IL-18 mRNA was amplified using reverse transcription (RT)(cDNA) and PCR. A representative lane is depicted from eachtransplant group and time point. Interestingly, the IL-18 PCRfindings mirror both the serum and heart IFN-g signal. As wasthe case with the serum IL-18, both the syngeneic (SYN) andallogeneic (ALLO) day 2 signals are depressed below control.The SYN transplants continue to show a lower signal until re-turning to control levels on day 14. In the ALLO group, thefindings are quite different. The ALLO level is three times thecontrol signal on day 6 and day 10, which correlates with theserum, splenocyte, and PCR IFN-g findings. The heart tissuesignal for IL-18 appears to be substantially higher in the ALLOgroups. Lane 1 is a size reference ladder, which does not un-dergo PCR. Negative PCR controls (not shown) were blank.(B) The histogram displays the relative intensity of the PCRsignal. Lane 1, Hyperladder size reference; lane 2, control (un-touched BALB/c heart tissue; lane 3, ALLO day 2; lane 4, SYNday 2; lane 5, ALLO day 6; lane 6, SYN day 6; lane 7, ALLOday 10; lane 8, SYN day 10; lane 9, ALLO day 14; lane 10,SYN day 14.
FIG. 6. (A) Agarose gel (2%) stained with ethidium bromide.RNA (1 mg) was isolated from each explanted heart, and theIFN-g signal was amplified using reverse transcription (RT)(cDNA) and PCR. A representative lane is shown from eachtransplant group and time point. Similar to the serum IFN-gELISA data and parallel to the IL-18 PCR findings, the signalfor IFN-g is higher in the allogeneic (ALLO) transplants. Ateach time point, the syngeneic (SYN) transplants demonstratean absent signal, similar to control tissue. Among the ALLOtransplants, the signal increases significantly by day 6, remainselevated through day 10, and then declines somewhat by day14. Lane 1 is a size reference ladder, which does not undergoPCR. Negative PCR controls (not shown) were blank. (B) Thehistogram displays the relative intensity of the PCR signal. Lane1, Hyperladder size reference; lane 2, control (untouchedBALB/c heart tissue); lane 3, ALLO day 2; lane 4, SYN day2; lane 5, ALLO day 6; lane 6, SYN day 6; lane 7, ALLO day10; lane 8, SYN day 10; lane 9, ALLO day 14; lane 10, SYNday 14.

time points after transplant. This finding correlates with theELISA data. The allogeneic transplants show marked inductionof IFN-g both at day 6 and day 10.
DISCUSSION
We have described the pattern of IL-12 and IL-18 produc-tion after murine allogeneic heart transplantation, particularlyin relation to clinical graft function and histologic rejection. Us-ing a combination of serum and splenocyte markers, we havedemonstrated a pronounced allogeneic IL-12/IL-18 responsethat correlates with rising IFN-g levels. This evidence is ac-companied by strong clinical and histologic evidence of graftrejection. The PCR data demonstrating a rising signal for IFN-g may arise from the graft-infiltrating cells that can be seen onhistologic study. Further, although the serum IL-18 and IFN-glevels appear to decline at day 10, the PCR findings suggestthat the graft continues to produce an increased mRNA signalfor IL-18 and IFN-g. These findings suggest a role for IL-12and IL-18 induction of IFN-g in murine allogeneic cardiactransplantation.
IL-18, originally known as IFN-g-inducing factor, is an 18-kDa cytokine, synthesized by Kupffer cells and activatedmacrophages.(18) The protein is a 157-amino acid polypeptidethat exerts biologic activity in a monomeric form.(18) The cDNAfor IL-18 has been cloned, and its product has been shown toinduce helper T cells (Th1), NK cells, and Fas ligand expres-sion.(5,6,18) IL-18 as a single agent, while inducing many of thesame responses attributable to IL-12, is thought to be a morepotent inducer of IFN-g than is IL-12.(18)
Actually, IL-12 and IL-18 act synergistically in the induc-tion of IFN-g.(12) IL-12 conditions T cells to respond to IL-18by increasing the expression of IL-18 T cell receptor,(12) andIL-18 potentiates the IL-12 induction of Th1 cells.(19,20) Al-though these two cytokines induce similar effects, their mech-anisms of action are distinct.(18) Monoclonal antibody to IL-12fails to abolish the immune effects of IL-18, and the IFN-g re-sponse to IL-12 is increased significantly in the presence of IL-18.(18)
Prior studies have demonstrated a dramatic synergy betweenIL-12 and IL-18 in IFN-g induction during stress, trauma, andinfection.(13–16) The present study describes the interaction ofthese factors in relation to the rejection response following car-diac transplantation. Further understanding of the complex in-teraction among IL-12, IL-18, and IFN-g may permit the de-velopment of new agents and treatments directed at the immuneresponse to allogeneic heart transplantation.
REFERENCES
1. RUSSEL, P.S., CHASE, C.M., WINN, H.J., and COLVIN, R.B.(1994). Coronary atherosclerosis in transplanted mouse hearts.Transplantation 57, 1367–1371.
2. SOKOLOWSKI, A., JENSEN, T., KOGLIN, J., and RUSSEL,M.E. (1998). Reduced transplant arteriosclerosis in murine cardiacallografts placed in interferon knockout recipients. Am. J. Pathol.152, 359–365.
3. WU, C.J., LOVETT, M., WONG-LEE, J., MOELLER, F., KITA-MURA, M., GORALSKI, T., BILLINGHAM, M.E., STARNES,V., and CLAYBERGER, C. (1992). Cytokine gene expression inrejecting cardiac allografts. Transplantation 54, 326–332.
4. ABBAS, A., LICHTMAN, A., and POBER, J. (1994). Cellular andMolecular Immunology, 2nd ed. Philadelphia: W.B. Saunders Com-pany.
5. USHIO, S., NAMBA, M., OKURA, T., HATTORI, K.,NUKADA, Y., AKITA, K., TANABE, F., KONISHI, K., MI-CALLEF, M., FUJII, M., et al. (1996). Cloning of the cDNA forhuman IFN-g-inducing factor, expression in Escherichia coli,and studies on the biologic activities of the protein. J. Immunol.156, 4274–4279.
6. TSUTSUI, H., NAKANISHI, K., MATSUI, K., HIGASHINO, K.,OKAMURA, H., MIYAZAWA, Y., and KANEDA, K. (1996).IFN-g-inducing factor up-regulates Fas ligand-mediated cytotoxicactivity of murine natural killer cell clones. J. Immunol. 157,3367–3973.
7. TRINCHIERI, G.M., MATSUMOTO-KOBAYASHI, S.C.,CLARK, J., and SEEHRA, L. (1984). Response of resting humanperipheral blood natural killer cells to interleukin-2. J. Exp. Med.160, 1147–1169.
8. WILSON, A.N., HARRIS, J.M., and COOMBS, R.R.A. (1988).Interleukin-2-induced production of interferon-g by resting humanT cells and large granular lymphocytes: requirement for accessorycell factors, including interleukin-1. Cell. Immunol. 113, 130–142.
9. OKAMOTO, I., KOHNO, K., TANIMOTO, T., IKEGAMI, H.,and KURIMOTO, M. (1999). Development of CD81 effector cellsis differentially regulated by IL-18 and IL-12. J. Immunol. 162,3202–3211.
10. MICALLEF, M.J., OHTSUKI, T., KOHNO, K., TANABE, F.,USHIO, S., NAMBA, M., TANIMOTO, T., TORIGOE, K., FIJII,M., IKEDA, M., et al. (1996). Interferon-g-inducing factor en-hances T helper 1 cytokine production by stimulated human T cells:synergism with interleukin-12 for interferon-g production. Eur. J.Immunol. 26, 1647–1651.
11. YOSHIMOTO, T., OKAMURA, H., TAGAWA, Y., IWAKURA,Y., and NAKANISHI, K. (1997). Interleukin-18 together with in-terleukin-12 inhibits IgE production by induction of interferon-gproduction from activated B-cells. Proc. Natl. Acad. Sci. USA 94,3948–3953.
12. AHN, H.-J., MARUO, S., TOMURA, J., MU, T., HAMAOKA,K., NAKANISHI, S., CLARK, M., KURIMOTO, H., OKAMURA,H., and FUJIWARA, H. (1997). A mechanism underlying synergybetween IL-12 and IFN-g-inducing factor in enhanced productionof IFN-g. J. Immunol. 159, 2125–2134.
13. SCOTT, P., and KAUFMANN, S.H.E. (1991). The role of T-cellsubsets and cytokines in the regulation of infection. Immunol. To-day 12, 346–348.
14. SHER, A., and COFFMAN, R.L. (1992). Regulation of immunityto parasites by T cells and T cell-derived cytokines. Annu. Rev.Immunol. 10, 385–409.
15. HEREMANS, H., VAN DAMME, J., DILLEN, C., DIJKMANS,R., and BILLIAU, A. (1990). Interferon-g is a mediator of lethallipopolysaccharide-induced Shwartzman-like shock reactions inmice. J. Exp. Med. 171, 1853–1869.
16. OKAMURA, H., NAGATA, K., KOMATSU, T., TANIMOTO, T.,NUKATA, Y., TANABE, F., AKITA, K., TORIGOE, K.,OKURA, T., FUKUDA, S., et al. (1995). A novel costimulatoryfactor for gamma interferon induction found in the livers of micecauses endotoxic shock. Infect. Immun. 63, 3966–3972.
17. CORRY, R.J., WINN, H.J., and RUSSEL, P.S. (1973). Primaryvascularized allografts of hearts in mice: the role of H-2D, H-2K,and non H-2 antigens in rejection. Transplantation 16, 343–350.
18. OKAMURA, H., TSUTSUI, H., KOMATSU, T., YUTSUDO, M.,
AFFLECK ET AL.8

HAKURA, A., TANIMOTO, T., TORIGOE, K., OKURA, T.,NUKADA, Y., HATTORI, K., et al. (1995). Cloning of a new cytokine that induces IFN-g production by T cells. Nature 378,88–91.
19. ROBINSON, D., SHIBUYA, K., MUI, A., ZONIN, F., MURPHY,E., SANA, T., HARTLEY, S.B., MENON, S., KASTELEN, R.,BAZAN, F., et al. (1997). IGIF does not drive Th1 developmentbut synergizes with IL-12 for interferon-g production and activatesIRAK and NFkB. Immunity 7, 571–581.
20. HSIEH, C.-S., MACATONIA, S.E., TRIPP, C.S., WOLF, S.F.,O’GARRA, A., and MURPHY, K.M. (1993). Development of Th1CD41 T cells through IL-12 produced by Listeria-inducedmacrophages. Science 260, 547–549.
Address reprint requests to:Dr. David A. Bull
Division of Cardiothoracic SurgeryUniversity of Utah Health Sciences Center
School of Medicine 3C127Salt Lake City, UT 84132
Tel: (801) 581-5311Fax: (801) 585-3936
E-mail: [email protected]
Received 11 August 2000/Accepted 16 August 2000
IL-18 PRODUCTION FOLLOWING TRANSPLANTATION 9