Inter-α-inhibitor in urine and calcium oxalate urinary crystals
Transcript of Inter-α-inhibitor in urine and calcium oxalate urinary crystals

British Journal of Urology (1998), 81, 20–26
Inter-a-inhibitor in urine and calcium oxalate urinary crystalsC.J. DAWS ON, P.K. GROVER and R.L. RYALLDepartment of Surgery, Flinders Medical Centre, Bedford Park, South Australia, Australia
Objective To determine which chains of inter-a-inhibitor As well as H1 and H2, the crystals from both sexescontained a protein band at #33 kDa. In many cases(IaI) are present in urine and whether they are also
found in calcium oxalate (CaOx) crystals generated in there appeared to be no direct relationship betweenthe proteins detected in the crystals and the urinehuman urine.
Materials and methods Fresh urine specimens were col- samples from which they were derived, which probablyreflects the well known instability of IaI and thelected from five women and five men with no previous
history of stone disease. An aliquot of each urine was occurrence of a range of bikunin fragments in urine.Conclusion These results show for the first time that H1retained for analysis, the remainder treated with a
standard load of oxalate and the CaOx crystals precipi- and H2 are present in human urine and urinary CaOxcrystals, that the bikunin chain of IaI is not the onlytated from each specimen demineralized with ethylene-
diamine tetracetic acid. The resulting organic extracts part of the molecule capable of participating in CaOxcrystallization in urine, and in theory at least, in thefrom crystals and their corresponding urine samples
were subjected to sodium dodecyl sulphate gel elec- regulation of crystallization events in stone formation.It is also apparent that significant fragmentation oftrophoresis analysis and Western blotting using a
commercial polyclonal antibody to IaI. IaI occurs both in vivo and in vitro, and this must beconsidered in any study attempting to elucidate theResults Heavy chain 1 (H1) and 2 (H2) of IaI were
commonly found in every urine sample, and in the influence of this protein in the formation of CaOxstones.CaOx crystals precipitated from those urine samples.
Several protein bands were visible in urine samples Keywords inter-a-inhibitor, bikunin, calcium oxalate,urolithiasis, urinefrom both sexes in the molecular mass range
25-70 kDa, which may be bikunin or its fragments.
leading to the formation of the calculus [1,4]. In theIntroductionpast, this dual origin of macromolecules severely con-founded the identification and characterization of individ-Proteins comprise by far the largest proportion of the
organic component known to be present in all human ual matrix components and the elucidation of theirpossible functions in stone pathogenesis; however, thiskidney stones [1], suggesting that they may play a
crucial role in stone pathogenesis. Although studied by has now been largely eliminated by the study of calciumoxalate (CaOx) crystals freshly precipitated from urine,Boyce and his colleagues in the 1950s (see [2]) real
progress in the analysis and unambiguous identification rather than stones themselves [4]. Such crystals containno macromolecules arising secondarily from cellularof individual proteins in stones has been made possible
only by recent advances in biochemical, immunological trauma and their study therefore permits an assessmentof the possible role of urinary macromolecules in theand physicochemical technology. A broad spectrum of
protein classes has been identified in stones, including crucial crystallization events leading to stone formation.One protein recently shown to be present in calciummucoproteins, serum proteins, and calcium-binding pro-
teins, and various individual members of these protein stones is inter-a-inhibitor (IaI) [5], a member of theKunitz-type serine proteinase inhibitor superfamily.groups have been, and continue to be, specifically ident-
ified [3]. However, the mere presence of a protein in Although IaI has been known by a variety of names, inthis paper the nomenclature proposed by Salier et al. [6]stones does not automatically equate to a specific func-
tion in stone formation, as matrix macromolecules can has been adopted to describe the protein and its relatives.Circulating in adult human plasma at a concentrationresult just as easily from cellular injury caused by the
stone itself as from participation in crystallization events of #240 mg/L [7], IaI is not a simple molecule. Theintact protein has a relative molecular mass (M
r) of
180–240 kDa [8] and comprises three distinct peptidesAccepted for publication 16 September 1997
20 © 1998 British Journal of Urology

INTER-a-INHIBITOR IN URINE AND CALCIUM OXALATE URINARY CRYSTALS 21
consisting of two heavy chains (H1 and H2) covalently reported that the protein derived from the urine of stoneformers inhibited CaOx crystallization less than the pro-linked by the glycosaminoglycan (GAG) chondroitin
sulphate (ChS) to a light chain, HI30, now most com- tein isolated from healthy subjects and speculated thatthis might reflect some alteration in the protein’s struc-monly known as bikunin [8]. Bikunin is found free in
plasma and is excreted in urine [9] where it reportedly ture [14]. Other findings also showed that fragments ofIaI inhibited the deposition of CaOx in inorganic milieux.undergoes further degradation [10] to fragments HI-14
and HI-8 (Fig. 1). The complexity of IaI is emphasized Tang et al. [15] isolated from urine two inhibitoryproteins with M
rs of 21 and 35 kDa, both of which hadby the fact that its three protein chains are encoded by
three genes on three diCerent chromosomes; the genes amino-acid sequence identity with bikunin. On the basisof its electrophoretic and elution properties they con-encoding H1, H2 and bikunin are on chromosomes 3,
10 and 9, respectively [8]. cluded that the 21 kDa protein was nephrocalcin [16],a protein long known to possess properties suggestive ofThe presence of IaI in stones reinforces previous results
which implicated the protein in stone formation. In a function in CaOx stone pathogenesis.Available evidence therefore indicates that the proteins1990, Sørensen et al. isolated from human urine a
protein that inhibited CaOx crystal growth in an inor- studied by all these authors were either bikunin itself,or a fragment of bikunin, a supposition supported in aganic crystallization system [11]. This protein had a M
rof #40 kDa and amino-acid sequencing suggested that recent report that UAP is indeed bikunin [17].
Furthermore, the potent inhibition of CaOx crystalit might be a relative of IaI [11]. Several years later,Atmani et al. isolated a similar urinary protein from growth by these proteins, coupled with the known
presence of bikunin and its fragments in urine, suggestedurine, which had a Mr
of 35 kDa and also inhibitedCaOx crystal growth under similar conditions [12]. the possible existence of a relationship between IaI and
CaOx stone formation. Although this possibility wasAnalysis showed that it was a glycoprotein containing8.5% carbohydrate and was rich in uronic acid. They strengthened by the demonstration that bikunin is pre-
sent in trace amounts in CaOx crystals precipitated fromnamed the protein uronic-acid-rich protein (UAP) andlater showed that it shared some amino-acid sequence normal human and rat urine [18] that study was
concerned with the association only between bikuninidentity with IaI [13]. Subsequently, the same authors
Fig. 1. Schematic representation of inter-a-inhibitor (IaI) and its fragments. Intact IaI consists of the two heavy chains H1, H2 linked viathe glycosaminoglycan, chondroitin sulphate, to the bikunin light chain. Bikunin can also fragment further into HI14 and HI8. Adaptedfrom [8].

22 C.J. DAWSON, P.K. GROVER and R.L. RYALL
and CaOx crystals. To our knowledge, the H1 and H2 sample volume was reduced to several millilitres bypolyethylene glycol (PEG), and the sample was thenchains of IaI have not been reported to be present in
urine, and consequently therefore their participation in filtered (0.2 mm MinisartA NML, Sartorius AG,Germany), applied to a 1×26 cm Bio-gel P-6 DG columnCaOx crystallization has not been studied. Thus the aims
of the present study were to determine which chains or (Bio-Rad) and desalted using distilled water. The proteinpeak was collected, lyophilized and stored at −20°Cfragments of the IaI molecule are present in urine from
normal subjects and to ascertain whether they are before analysis by SDS-PAGE.associated with CaOx crystals precipitated from thoseurine samples.
Generation and demineralization of CaOx crystals
Using a method described previously [4], CaOx crystalsMaterials and methodswere precipitated from each urine specimen by addingoxalate in suBcient quantities (determined by titration)
Chemicalsto induce spontaneous crystallization detectable by aCoulter Counter. Sodium oxalate solution was added atAll reagents used were of analytical grade and all
solutions prepared with high purity water (Permutit 0, 1 and 2 h, and samples incubated in a shaking waterbath at 37°C for a total of 4 h. The crystals wereAustralia, Brookvale, NSW, Australia). Unless stated
otherwise, biochemicals were supplied by Sigma harvested by Millipore filtration (0.22 mm), washed thor-oughly with distilled water and lyophilized before storageChemical Co, St Louis, MO, USA. Other reagents were
obtained from the following sources: acrylamide, N,N’- at −20°C.The crystals (50 mg) were demineralized by stirringmethylene-bis-acrylamide, b-mercaptoethanol, N,N,N∞,
N∞-tetramethylethylenediamine (TEMED) (Bio-Rad in 4 mL of 0.25 mol/L EDTA solution (pH 8.0) for #2 hat room temperature. The samples were then filteredLaboratories, Richmond, CA 94804, USA); glycerol,
hydrogen peroxide (BDH Chemicals Australia, Kilsyth, and desalted using the Bio-gel P-6 DG desalting columnas described above. The protein peak was collected andVic, Australia); glycine, sodium acetate, sodium thio-
sulphate, ethylenediamine-tetracetic acid (EDTA), meth- lyophilized, and the entire sample dissolved in 100 mL ofSDS-PAGE reducing sample buCer (0.06 mol/L Trisanol, potassium di-hydrogen orthophosphate (Ajax
Chemicals, Auburn, NSW, Australia); bromophenol blue, pH 6.8, 10% glycerol, 2% SDS, 5% b-mercaptoethanoland 0.01% bromophenol blue) in preparation for SDS-glutaraldehyde (Merck, Darmstadt, Germany), di-
potassium hydrogen orthophosphate (Fisons Scientific PAGE.Equipment, Loughborough, England).
Purification and fragmentation of standard IaICollection of urine samples
Intact IaI was purified from expired samples ofProthrombinex-HT, a concentrate of human bloodUrine samples from five healthy men (aged
33–49 years) and five healthy women (aged coagulation factors (manufactured from human plasmaby CSL Ltd, Victoria, Australia). Fragments of the protein21–48 years) with no previous history of stone disease
were collected without preservative. For the duration of were used as standards for SDS-PAGE and Westernblotting. The purification of intact IaI, generation of itscollection (#24 h) and before use, the urine samples
were stored at 4°C. The absence of blood was confirmed fragments by alkaline hydrolysis and determination ofits tryptic inhibitory activity are described in detailusing Multistix test strips (Miles Laboratories, Mulgrave,
Victoria, Australia). Each specimen was centrifuged at elsewhere [5].6100×g for 20 min at 20°C in a Beckman J2–21 M/Ecentrifuge (Beckman Instruments, Palo Alto, CA, USA)
SDS-PAGE and Western blottingand the supernatant filtered through 0.22 mm Milliporefilters (# GVWP 14250, Millipore Corporation, Bedford, Urine samples and protein extracts obtained from CaOx
crystals generated in human urine were analysed byMA, USA).SDS-PAGE and Western blotting as described elsewhere[5]. IaI and related fragments on blots were detected
Preparation of urinary proteins for electrophoresisusing a rabbit antihuman IaI (15100 dilution, code noA301, Dako Corporation, CA, USA) followed by theAbout 200 mL of each sample was dialysed (cellulose
seamless dialysis tubing #453138, nominal molecular addition of peroxidase-conjugated goat antirabbit IgG ata dilution of 152000 (catalogue no. 170–6515, Bio-weight threshold 6000–8000, Union Carbide, Ill, USA)
overnight at 4°C against 5 L of distilled water. The Rad, CA, USA).

INTER-a-INHIBITOR IN URINE AND CALCIUM OXALATE URINARY CRYSTALS 23
Results
Fragments of IaI resulting from alkaline hydrolysis areshown in the right-hand lanes of the gels and Westernblots shown in Figs 2 and 3. The two sharp bands withM
rs corresponding to 77 and 82 kDa probably corre-
spond to the two heavy chains of the protein, H1 andH2, respectively. Other bands with higher M
rs were also
apparent, one of which may correspond to anotherheavy chain, H3, which is closely related to H1 and H2,has a M
rof 90 kDa, and is known to associate with
HI30 in plasma to form a complex known as pre-a-trypsin inhibitor [8]. Additional bands with M
rs of
20–40 kDa can also be seen. These may represent theIaI light chain, bikunin, or its degradation products,although it is not possible to confirm this without aminoacid sequence analysis. Several protein bands can also
a
b
Fig. 3. a, SDS-PAGE gel stained with silver illustrating Mr
standards(STD), normal female urinary proteins (U) and the matrix extractedfrom CaOx crystals generated in undiluted female urine (C).Degradation products of alkaline hydrolysis of intact IaI are in thelane marked IaI. b, Western blot of normal female urinary proteins(U) and the matrix extracted from CaOx crystals generated inundiluted female urine (C) immunoblotted with antihuman IaIantibody and detected by DAB. M
rstandards (STD). Degradation
products of alkaline hydrolysis of intact IaI are in the lanemarked IaI.
be seen in the range 10–20 kDa, which may be theHI14 or HI8 peptides reportedly resulting from fragmen-tation of IaI in vitro [10].
Urine samples and crystals from men and women
Figure 2a shows the SDS-PAGE patterns of the proteinspresent in the urine samples from healthy men, togetherwith those found in the corresponding organic EDTAextracts of the crystals precipitated from each urine. It
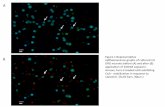
a
bis apparent that relatively few of the many proteinsFig. 2. a, SDS-PAGE gel stained with silver illustrating M
rstandards
(STD), normal male urinary proteins (U) and the matrix extracted present in the urine samples are associated with thefrom CaOx crystals generated in undiluted male urine (C). CaOx crystals precipitated from them. Figure 2b showsDegradation products of alkaline hydrolysis of intact IaI are in the the Western blots corresponding to the gels in Fig. 2a;lane marked IaI. b, Western blot of normal male urinary proteins inter-individual diCerences are apparent. Bands pre-(U) and the matrix extracted from CaOx crystals generated in
sumed to be H1 (77 kDa) and H2 (82 kDa) can be seenundiluted male urine (C), immunoblotted with antihuman IaIin all the urine samples and their associated organicantibody and detected by DAB. M
rstandards (STD). Degradation
crystal extracts. Several bands, some or all of which mayproducts of alkaline hydrolysis of intact IaI are in the lanemarked IaI. represent bikunin or its fragments, can be seen at M
rs

24 C.J. DAWSON, P.K. GROVER and R.L. RYALL
ranging from 25 to 47 kDa. Urine samples 1, 2 and 5 course of stone formation. On the other hand, theirdetection in CaOx crystals precipitated from urine, orshow double bands at #49–57 kDa and at #25 kDa,
while there are similar doublets in urine samples 3 and demonstration that their inhibitory potency is retainedin urine, would constitute more convincing, although4, although in the lower range 25–17 kDa. These two
lower Mr
bands may be the HI14 and HI8 fragments, not conclusive, support for the belief that IaI may beinvolved in the pathogenesis of renal calculi.respectively, of bikunin. Faint bands with M
rs greater
than those of H1 and H2, which may represent H3, The results of this study have confirmed previousreports that alkaline hydrolysis causes significant frag-were also seen in urine samples 1, 2 and 5, but not in
the corresponding crystals. As well as H1 and H2, a mentation of the IaI molecule [20]. Western blots ofSDS-PAGE gels of urinary proteins derived from healthyband at #33 kDa was consistently detected in the
crystals, which, in samples 1, 2 and 5, could represent men and women clearly showed the presence of assortedbands with M
rs of 20–80 kDa, which reacted with thefragments of the peptides visible at #49–57 kDa, or at
#70 kDa, in their corresponding urine samples. polyclonal IaI antibody. Some interindividual variationin the pattern of protein bands was visible amongst theHowever, this possibility is negated because the crystals
precipitated from urine samples 3 and 4 also contained male and female urine samples, and diCerences betweenthe sexes were also obvious. Without antibodies tothe #33 kDa protein, despite the complete absence of
bands at 49–57 kDa in the samples themselves. specific regions of the IaI protein chains it was notpossible to identify positively all these bands, althoughFigure 3a shows the SDS-PAGE of the proteins present
in the urine of the female subjects and those in the CaOx some of them almost certainly corresponded to bikuninor its fragments HI14 and HI8, which are known to becrystals precipitated from them. Western blots of these gels
(Fig. 3b) show that, as was seen with the men, the H1 present in human urine [10].These results show that the urinary excretion of IaIand H2 chains were common, although they were not
detected in the organic matrix of crystals from urine fragments is variable, gender-dependent and highly com-plex; the component chains, which can degrade tosamples 2, 3 and 4. A band at 41–45 kDa, which may
represent bikunin, was visible in all the female urine smaller fragments, appear capable of associating in avariety of combinations, the products of which may orsamples and as also occurred with the crystals from the
male urine samples, a band at #33 kDa was consistently may not be linked to ChS. Even bikunin, the simplestprotein chain of IaI, has proved remarkably variable,visible (Fig. 3b). A prominent band at #69 kDa was also
seen in all the female urine samples. Only urine sample 1 with its Mr
reportedly ranging from as low as 20 kDa[21] to as high as 70 kDa [22]. Whether or not theshowed evidence of bands with M
rs greater than H1 and
H2, one of which was very faintly visible in the correspond- protein is linked to the ChS moiety introduces furthervariation, as does its source; human bikunin deriveding crystals. None of the female urine samples contained
the band at around 25 kDa which was visible in all the from plasma has been reported to have a Mr
of 35 kDaon SDS-PAGE, while that from urine, estimated undermale urine samples, although a band with a similar M
rwas seen in the crystals precipitated from sample 5. identical conditions, runs at 45 kDa [17].
As far as we are aware, the urinary excretion of theheavy chains of IaI has not hitherto been reported. ThisDiscussionprobably results more from a historical preoccupationwith the bikunin portion of the protein and a consequentA relationship between IaI and urine was first noted as
long ago as 1909 when Bauer and Reich [19] showed lack of interest in H1 and H2, rather than failed attemptsto detect them. Previously, the isolation or detection ofthat human urine inhibited the action of trypsin, a
property that is now recognized to be attributable to proteins related to IaI in urine involved either highlyspecific procedures [11–13,15], which would not havewhat has since become known as IaI. More recently,
results of several studies have suggested that IaI deriva- allowed the isolation of the heavy chains, or the measure-ment of urinary antitrypsin activity [23], which is con-tives may be associated with urinary stone formation by
virtue of their ability to inhibit the deposition of CaOx fined to the bikunin moiety of the molecule [24].Furthermore, bikunin itself is known to be active against[11–15,17]. However, this inhibitory activity has been
shown to occur only in inorganic crystallization systems, several proteases [25] and is the only portion of IaI bothto have been isolated from urine, and shown to inhibitwhich are widely acknowledged as inadequate models
for stone formation, as they do not necessarily allow the CaOx crystallization [11–15,17]. It has been widelyassumed therefore that bikunin is the only part of IaIeCects that an inhibitor would show in urine to be
reproduced. Therefore, the reported inhibitory activity of excreted in urine. However, in the present study, twoclear protein bands corresponding to M
rs of 77 andurinary IaI derivatives cannot be regarded as absolute
evidence that the protein or its fragments influence the 82 kDa, which almost certainly represent the H1 and

INTER-a-INHIBITOR IN URINE AND CALCIUM OXALATE URINARY CRYSTALS 25
H2 chains of IaI, were a consistent finding in all the the urine samples and it is possible that these representedcomplexes of bikunin with other chains or derivatives ofurine samples from both sexes, and in several urine
samples a faint band, possibly representing H3, was also IaI that had fragmented during the crystallization processto release the bikunin moiety, which had subsequentlyevident. In addition, several other fragments which may
represent various combinations of bikunin, with and been incorporated into the crystals. Alternatively, com-plexes containing bikunin may have broken down as awithout the ChS moiety, were also detected, although
not as consistently as H1 and H2. result of proteolysis during incubation after the additionof the oxalate load, which itself may also have causedThe presence of H1 and H2 in urine suggests that
they are actively secreted by the kidney and immunohis- release of bikunin.In marked contrast, H1 and H2 were consistentlytochemical studies have shown that IaI is present in the
proximal convoluted tubules of the human kidney [26]. present in most of the urine samples and crystals.Although trace quantities of bikunin are present in CaOxIt is unclear whether the intact IaI molecule is located
in the kidney, or only individual chains or fragments, as crystals precipitated from human urine [18], that studydid not report the presence of H1 or H2 because thethe antibody used in those studies [26], which was the
same as that used here, cross-reacts with the parent IaI antibody used was specific for bikunin. Another study[23] measured the trypsin inhibitory activity of urinemolecule and all of its recognized derivatives, including
bikunin, H1, and H2. and did not report the presence of the heavy chains,presumably because they are unrecognizable by theWith the exception of Tamm-Horsfall glycoprotein,
whose inhibitory potency probably results from steric crossed immunoelectrophoresis technique which wasused to detect bikunin.hindrance [27], it is generally acknowledged that macro-
molecules inhibit growth and aggregation by binding to The present study shows that the putative role of IaIin stone formation is more complex than has beenthe crystal surfaces. Unless inhibition is complete, bound
macromolecules will become embedded within the crystal- previously supposed. Several IaI derivatives are found inhuman urine, relatively few of which are incorporatedline architecture in the face of overwhelming supersat-
uration. CaOx crystals precipitated from human urine into CaOx crystals; those not present in crystals are mostunlikely to have any influence in stone formation. Thecontain, in comparison to the many present in urine
itself, relatively few proteins whose incorporation into the presence of what is presumably a form of bikuninsupports previous findings suggesting that this portionstructure is the result of selective binding (see [2]). The
present study showed that derivatives of IaI are present of the IaI molecule might influence CaOx crystallizationin urine, although it cannot be assumed that it fulfilsin CaOx crystals generated from the urine of healthy men
and women. In particular, a protein at #33 kDa was some role in stone pathogenesis. Moreover, that it ispresent in only small amounts and cannot be consistentlyconsistently found in crystals from both sexes, which is
in keeping with another report that a 35 kDa form of detected in calcium stones [5] suggests that such a role,if it exists, is probably minor. Nonetheless, the unexpec-bikunin is present in trace amounts in CaOx crystals
precipitated from both human and rat urine [18]. A ted detection of both heavy chains of IaI in urine andCaOx crystal matrix, as well as in calcium stones [5],second band at #45 kDa was also infrequently seen.
Both of these may be diCerent forms of bikunin, with the has introduced wider possibilities for a potential relation-ship between IaI and stone formation, as it is conceivablelower M
rband deriving from degraded IaI in filtered
plasma, and the heavier band representing the urinary that they may influence crystallization and therefore, atleast in theory, stone development. Further studies areform of the molecule isolated by Sørensen et al. [11].
Certainly, the broad electrophoretic patterns of the bands now required to test the individual eCects of bikunin, H1and H2 on CaOx crystallization in urine before it will bewithin the M
rrange for bikunin in urine and some
crystals were similar in appearance to the diCuse zones possible to assess their function, if any, in urolithiasis.shown by Balduyck et al. [28] for bikunin.
Because of the complex and unstable nature of IaI Acknowledgementsand its derivatives, the variation in the urinary bikunincontent between the sexes and between individuals of This project was supported in part by grant no. 940283
from the National Health and Medical Research Councilthe same sex was not surprising. What was unexpectedwas the consistent mismatch between the protein con- of Australia.tent of the CaOx crystals and the urine from which theyhad been generated. All crystals contained a 33 kDaband, which was almost certainly bikunin and could not Referencesbe detected in the corresponding urine samples. However, 1 Boyce WH. Organic matrix of human urinary concretions.
Am J Med 1968; 45: 673–83in most cases, bands with higher Mrs were present in

26 C.J. DAWSON, P.K. GROVER and R.L. RYALL
2 Ryall RL, Stapleton AMF. Urinary macromolecules in 17 Atmani F, Mizon J, Khan S. Identification of uronic-acid-calcium oxalate stone and crystal matrix: Good, bad, or rich protein as urinary bikunin, the light chain of inter-a-indiCerent? In Khan SR, ed. Calcium oxalate in biological inhibitor. Eur J Biochem 1996; 236: 984–90systems. Boca Raton: CRC Press, 1995: 265–90 18 Atmani F, Opalko FJ, Khan SR. Association of urinary
3 Ryall RL. Glycosaminoglycans, proteins, and stone forma- macromolecules with calcium oxalate crystals induced intion: adult themes and child’s play. Pediatr Nephrol 1996; vitro in normal human and rat urine. Urol Res 1996;10: 656–66 24: 45–50
4 Doyle IR, Ryall RL, Marshall VR. Inclusion of proteins into 19 Gebhard W, Hochstrasser K. Inter-a-trypsin inhibitor andcalcium oxalate crystals precipitated from human urine: a its close relatives. In Barrett AJ, Salvesen G, eds, Proteinasehighly selective phenomenon. Clin Chem 1991; 37: inhibitors. Amsterdam: Elsevier Science Publishers, 1986:1589–94 390–401
5 Dawson CJ, Grover PK, Kanellos J et al. Inter-a-inhibitor in 20 Michalski C, Piva F, Balduyck M et al. Preparation andcalcium stones. Clinical Science 1997; Submitted properties of a therapeutic inter-alpha-trypsin inhibitor
6 Salier JP, Rouet P, Raguenez G, Daveau M. The inter-a- concentrate from human plasma. Vox Sang 1994; 67:inhibitor family: from structure to regulation. Biochem J 329–361996; 315: 1–9 21 Balduyck M, Laroui S, Mizon C, Mizon J. A proteoglycan
7 Mizon C, Balduyck M, Albani D, Michalski C, Burnouf T, related to the urinary trypsin inhibitor (UTI) links the twoMizon J. Development of an enzyme-linked immunosorbant heavy chains of inter-a-trypsin inhibitor. Biol Chem Hoppe-assay for human plasma inter-a-trypsin inhibitor (ITI) Seyler 1989; 370: 329–36using specific antibodies against each of the H1 and H2 22 Proksch G, Routh J. The purification of the trypsin inhibitorheavy chains. J Immunol Meth 1996; 190: 61–70 from human pregnancy urine. J Lab Clin Med 1972;
8 Salier JP. Inter-a-trypsin inhibitor: emergence of a family 79: 491–9within the Kunitz-type protease inhibitor superfamily. TIBS 23 Odum L, Hansen-Nord G, Byrjalsen I. Human inter-a-1990; 15: 435–9 trypsin inhibitor and immunologically related inhibitors
9 Proksch GJ, Lane J, Nordschow CD. Interrelation of the investigated by quantitative immunoelectrophoresis. II.urinary trypsin inhibitor to human plasma inter-alpha-
Pathological conditions. Clin Chim Acta 1987; 162: 189–98trypsin inhibitor. Clin Biochem 1973; 6: 200–6
24 Morii M, Travis J. The reactive site of human inter-a-10 Hochstrasser K, Wachter E. Kunitz-type proteinase inhibi-
trypsin inhibitor is in the amino-terminal half of thetors derived by limited proteolysis of Inter-a-trypsin inhibi-
protein. Biol Chem Hoppe-Seyler 1985; 366: 19–21tor, I. Determination of the amino acid sequence of the
25 Potempa J, Kwon K, Chawla R, Travis J. Inter-a-trypsinantitryptic domain by solid-phase Edman degradation. Biol
inhibitor. Inhibition spectrum of native and derived forms.Chem Hoppe-Seyler 1979; 30: 1285–96J Biol Chem 1989; 264: 15109–1411 Sørensen S, Hansen K, Bak S, Justesen SJ. An unidentified
26 Odum L. Immunohistochemical investigation of inter-a-macromolecular inhibitory constituent of calcium oxalatetrypsin inhibitor in the urinary tract. APMIS 1989;growth in human urine. Urol Res 1990; 18: 373–997: 357–6012 Atmani F, Lacour B, Drueke T, Daudon M. Isolation and
27 Ryall RL, Harnett RM, Hibberd CM, Edyvane KA, Marshallpurification of a new glycoprotein from human urineVR. ECects of chondroitin sulphate, human serum albumininhibiting calcium oxalate crystallization. Urol Res 1993;and Tamm-Horsfall mucoprotein on calcium oxalate crys-21: 61–6tallization in undiluted human urine. Urol Res 1991;13 Atmani F, Lacour B, Strecker G, Parvy P, Drueke T,19: 181–8Daudon M. Molecular characteristics of uronic-acid-rich
28 Balduyck M, Mizon C, Loutfi H, Richet C, Roussel P, Mizonprotein, a strong inhibitor of calcium oxalate crystallizationJ. The major human urinary trypsin inhibitor is ain vitro. Biochem Biophys Res Commun 1993; 191: 1158–65proteoglycan. Eur J Biochem 1986; 158: 417–2214 Atmani F, Lacour B, Jungers P, Drueke T, Daudon M.
Reduced inhibitory activity of uronic-acid-rich protein inurine of stone formers. Urol Res 1994; 22: 257–60
15 Tang Y, Grover PK, Moritz RL, Simpson RJ, Ryall RL. IsAuthorsnephrocalcin related to the urinary derivative (bikunin) ofC.J. Dawson, PhD Student.inter-a-trypsin inhibitor? Br J Urol 1995; 76: 425–30P.K. Grover, Senior Research OBcer.16 Nakagawa Y, Abram V, Kezdy FJ, Kaiser ET, Coe FL.R.L. Ryall, Professor and Chief Medical Scientist.Purification and characterization of the principal inhibitorCorrespondence: Professor R.L. Ryall, Department of Surgery,of calcium oxalate monohydrate crystal growth in human
urine. J Biol Chem 1983; 258: 12594–600 Flinders Medical Centre, Bedford Park, SA 5042, Australia.






![Ιωάννης Γ. Γριβέας,MD,PhD · Transtubular Gradient (TTKG) Useful to assess the renal response to or serum K+ TTKG = [urine + (urine Osm/ Plasma Osm)] Plasma K+ Should](https://static.fdocument.org/doc/165x107/5e3548c75e633f0bc503cf7c/-mdphd-transtubular-gradient-ttkg-useful-to.jpg)










![The influence of γ O and Na on the formation of calcium silicate … · 2007. 2. 27. · Calcium silicate hydrates in the CaO quartz H 2 O system with C/S = 0.66 187 Luke [26] established](https://static.fdocument.org/doc/165x107/60cc12a7d6f169767d3b011c/the-influence-of-o-and-na-on-the-formation-of-calcium-silicate-2007-2-27.jpg)