Improved synthesis and in vitro evaluation of the cytotoxic profile of oxysterols oxidized at C4...
Transcript of Improved synthesis and in vitro evaluation of the cytotoxic profile of oxysterols oxidized at C4...

lable at ScienceDirect
European Journal of Medicinal Chemistry 70 (2013) 558e567
Contents lists avai
European Journal of Medicinal Chemistry
journal homepage: http: / /www.elsevier .com/locate/ejmech
Original article
Improved synthesis and in vitro evaluation of the cytotoxic profileof oxysterols oxidized at C4 (4a- and 4b-hydroxycholesterol) and C7(7-ketocholesterol, 7a- and 7b-hydroxycholesterol) on cells of thecentral nervous system
Thomas Nury a, Mohammad Samadi b,1, Amira Zarrouk a,c, Jean Marc Riedinger d,Gérard Lizard a,*
aUniversité de Bourgogne, Equipe ‘Biochimie du peroxysome, inflammation et métabolisme lipidique’ EA 7270, INSERM, Dijon, Franceb LCPMC-A2, ICPM, Département de Chimie, Université de Lorraine, 1 Bd Arago, Metz Technopôle, Metz, FrancecUniversité de Monastir, Faculté de Médecine, Laboratoire de Biochimie, LR12ES05 Lab-NAFS ‘Nutrition e Functional Food & Vascular Health’,Avenue Avicenne, Monastir, TunisiadCentre de Lutte Contre le Cancer GF Leclerc, Laboratoire de Biologie Médicale, Dijon, France
a r t i c l e i n f o
Article history:Received 3 May 2013Received in revised form5 September 2013Accepted 8 September 2013Available online 23 October 2013
Keywords:4a-Hydroxycholesterol4b-Hydroxycholesterol158N murine oligodendrocytesC6 rat glioma cellsSK-N-BE human neuroblastoma cellsGlial cells primary cultureCell viabilityCell growth
* Corresponding author. Université de Bourgogne, EINSERM, Faculté des Sciences Gabriel, 6 Bd Gabriel, 2380 39 62 56; fax: þ33 380 39 62 50.
E-mail addresses: mohammad.samadi@[email protected] (G. Lizard).
1 Tel.: þ33 387 54 77 93.
0223-5234/$ e see front matter � 2013 Elsevier Mashttp://dx.doi.org/10.1016/j.ejmech.2013.09.028
a b s t r a c t
Whereas the biological activities of oxysterols oxidized at C7 (7-ketocholesterol (7KC), 7b-hydrox-ycholesterol (7b-OHC), 7a-hydroxycholesterol (7a-OHC)) are well documented, those of oxysterolsoxidized at C4 (4b-hydroxycholesterol (4b-OHC), 4a-hydroxycholesterol (4a-OHC)) are not well known,especially on the cells of the central nervous system. Therefore, an improved methodology has beenvalidated for 4b-OHC and 4a-OHC synthesis, and the effects on cell viability and cell growth of thesemolecules were studied on immortalized, tumoral and normal brain cells (158N, C6 and SK-N-BE cells,and mixed primary cultures of astrocytes and oligodendrocytes). Whereas inhibition of cell growth with7KC, 7b-OHC, and 7a-OHC is associated with a decrease of cell viability (cytotoxic activities), our dataestablish that 4b-OHC and 4a-OHC have no effect on cell viability, and no or minor effect on cell growthevocating cytostatic properties. Thus, comparatively to oxysterols oxidized at C7, the toxicity of oxy-sterols oxidized at C4 is in the following range of order: 7KC � 7b-OHC > 7a-OHC > (4b-OHC � 4a-OHC).Interestingly, to date, 4b-OHC and 4a-OHC are the only oxysterols identified with cytostatic propertiessuggesting that these molecules, whereas not cytotoxic, may have some interests to counteract cellproliferation.
� 2013 Elsevier Masson SAS. All rights reserved.
1. Introduction
Cholesterol is an important structural element of cell mem-branes and it is also an essential substrate for biosynthesis ofnumerous molecules such as bile acids, steroid hormones andneurosteroids, which are potent and effective neuromodulatorsthat are synthesized from cholesterol in the brain [1e5]. Moreover,the cholesterol molecule is easily oxidized and may be transformedinto numerous oxidation products known as oxysterols. Thus,
quipe Bio-peroxIL (EA 7270),1000 Dijon, France. Tel.: þ33
aine.fr (M. Samadi), Gerard.
son SAS. All rights reserved.
oxysterols are 27-carbon-atom cholesterol oxidation productswhich can be produced endogenously by enzymatic reactions or byautoxidation [6e8]. They also can be provided by food. The enzy-matic pathways can form both A-ring, B-ring and side-chain hy-droxylated oxysterols depending on the enzyme and the tissue,while the non-enzymatic pathways form mainly B-ring oxysterols[8,9]. In human circulation, the quantitatively dominating oxy-sterols are 27-hydroxycholesterol, 24-hydroxycholesterol, 7a-hydroxycholesterol and 4b-hydroxycholesterol (4b-OHC) [10,11].
To date, there are some evidences that oxysterols contribute tothe regulation of numerous biological activities including choles-terol homeostasis, inflammation, cell differentiation, and prolifer-ation, and that they are also involved in the development ofdifferent pathologies such as osteoporosis, age-related maculardegeneration, cardiovascular and neurodegenerative diseases likeAlzheimer, Parkinson, and multiple sclerosis [12,13]. The oxysterols

T. Nury et al. / European Journal of Medicinal Chemistry 70 (2013) 558e567 559
also participate in the Hedgehog and wnt signaling pathways[14,15]. They are also important components of lipid rafts, andpotent activators of associated metabolic pathways [16e18]. Theability of some oxysterols, such as 7b-hydroxycholesterol, to inducecell death on different cancerous cells [12,19,20] leads to designnew anti-tumoral drugs, and to precise the roles of these moleculeson different types of cancer cells [20e23]. On the basis of theoxidation state on rings A and B of the sterol nucleus, and on theoxygenated groups known to be essential for cytotoxicity in naturaloccurring oxysterols, synthesized oxysterols were evaluated forcytotoxicity in several cancer and noncancerous cells [24,25].Among these molecules, 4b-hydroxycholesterol was studied andminor cytotoxic effects were observed on HT-29 cells deriving fromhuman colorectal adenocarcinoma [25]. In vivo, 4b-hydrox-ycholesterol is formed from cholesterol by cytochrome P4503A4(CYP3A4) and cytochrome P4503A5 (CYP3A5) [26,27] while itsisomer 4a-hydroxycholesterol would be produced by cholesterolautoxidation [27,28]. The cytotoxic activity of 4b-hydrox-ycholesterol, which is a potent Liver X Receptor a (LXRa) and LiverX Receptor b (LXRb) agonist [29,30], and which can thereforecontribute to the regulation of numerous genes [31], remainshowever not well known on normal or tumoral cells of the centralnervous system (CNS), which can constitute potential targets ofthese compounds when the blood brain barrier is altered either inpathological conditions or under the action of different physical orchemical treatments.
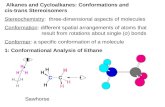
Therefore, in the present investigation an improved methodol-ogy is describe to synthetize 4b-hydroxycholesterol (4b-OHC), andits isomer 4a-hydroxycholesterol (4a-OHC), and the ability of thesemolecules to induce cell death and to modulate cell proliferationwas studied on different immortalized, tumoral and normal cells ofthe CNS (158N murine oligodendrocytes immortalized with the
Fig. 1. Synthesis of ring B oxygenated sterols at C4 position: 4a-hyd
SV40 large T-antigen [32,33], C6 rat glioma cells, SK-N-BE humanneuroblastoma cells, and mixed murine primary culture of glialcells (astrocytes and oligodendrocytes)). In those conditions, theeffects of 4b-OHC and 4a-OHC were compared with those of oxy-sterols known to have strong cytotoxic activities: 7-ketocholesterol(7KC) and 7b-hydroxycholesterol (7b-OHC) [12,13].
As the biological activities of these compounds are not wellknown [30], and as there is a growing interest for oxysterols andoxysterol derivatives for cancer treatment [34], the effects of 4b-OHC and 4a-OHC on cell viability and cell growth were determinedon various normal and tumoral cells of the CNS comparatively tooxysterols oxidized at C7. Indeed, 7b-OHC is a potent inducer of celldeath on C6 rat glioma cells [20]. Noteworthy, at the opposite ofoxysterols oxidized at C7 (mainly 7KC and 7b-OHC), and in agree-ment with data obtained on other cell types [19,24,25], our studydoes not show cytotoxic activities (induction of cell death associ-ated with reduced cell growth) of 4b-OHC and 4a-OHC onimmortalized, tumoral, and normal cells of the CNS used. However,it reveals cytostatic activities (absence of cell death associated withreduced cell growth) in a range of concentration from 5 to 160 mM,especially on 158N and C6 cells, and no effect on normal glial cells,suggesting that 4b-OHC and 4a-OHC may have some interests tocounteract cell proliferation.
2. Results and discussion
2.1. Chemistry
The reaction of SeO2 with cholesterol was reported to produce4b-hydroxycholesterol as the only product [35,36]. Recently, Ghoshet al. have reported that the oxidation of cholesterol using excess ofSeO2 produced the 4b-hydroxycholesterol 1 and 3b,4b,7a-
roxycholesterol (4a-OHC) and 4b-hydroxycholesterol (4b-OHC).

T. Nury et al. / European Journal of Medicinal Chemistry 70 (2013) 558e567560
dihydroxycholesterol [37]. In our hands, treatment of cholesterolwith SeO2 (1.5 equiv) in CHCl3 produced 4b-hydroxycholesterol 1and the 7a-hydroxycholesterol [38] 4 in 62% and 17%, respectively.It is important to mention that the formation of compound 4 fromcholesterol by SeO2 has not been reported previously. In contrast,treatment of cholesteryl acetate 5 with the SeO2 furnished 4b-hy-droxylated 6 and 6a-hydroxylated 7 in 50% and 25% respectively[39e41].
Surprisingly, treatment of cholesteryl acetate 5with SeO2 in thepresence of N-methylmorpholine (pKb ¼ 7.38) at 70 �C decreasesthe formation of compound 7 to 5% and increases the amount ofcompound 6 to 60% yield, whereas no reaction occurred in thepresence of triethylamine (pKb ¼ 11). These results show that theformation of 7 is dependent on the reaction medium (SeO2,pKa ¼ 2.62), and it seems that the formation of 6a-hydroxylated 7was produced from 4b-hydroxylated 6 rather than by oxidation ofcholesteryl acetate 5with SeO2 (the mechanism of the formation of6a-hydroxylated 7 from cholesteryl acetate 5 is currently understudy in our laboratory).
Finally, the 4a-hydroxycholesterol 2 was obtained as followed:catalytic oxidation of compound 6 with tetrapropylammoniumperruthenate (TPAP) [42] in the presence of 4-methylmorpholineN-oxide (NMO) afforded the expected ketone 8 in very goodyields, which was reduced selectively by NaBH4 and in situ hy-drolysis of the remaining acetate gave the 4a-hydroxyl cholesterol2 [43] (Fig. 1).
2.2. Structure activity relationship on cell viability and cell growth
On the basis of the oxidation state at C4 and C7 on ring A andB, respectively, of the sterol nucleus and on the oxygenatedgroups known to be essential for the side effects of differentoxysterols, various oxysterols were evaluated for their cytotox-icity (effects on cell viability and cell growth). The purpose of thisstudy was to evaluate the effects of 4a-OHC and 4b-OHC, whichare not well known, comparatively to oxysterols with welldescribed biological activities (7KC, 7b-OHC, and 7a-OHC) [13] oncells of the CNS.
Therefore, to evaluate the impact of the hydroxyl at C4, and theincidence of its position (a or b), a panel of immortalized, tumoraland normal cells of the CNS was used: murine oligodendrocytes158N immortalized with the SV-40 large T-antigen [44]; C6 ratglioma cells; SK-N-BE human neuroblastoma cells; mixed murineprimary culture of astrocytes and oligodendrocytes. The neuro-blastoma derived cell line (SK-N-BE) was chosen because of the roleof cholesterol and of a particular oxysterol, 24S-hydroxycholesterol,in the normal brain cellular functions and also because of the in-fluence of oxysterol imbalance in neurodegenerative processes[45]. Moreover, neuroblastoma as well as glioma (taken inconsideration in the present study with the use of C6 rat gliomacells) are common malignancies in childhood [46] with generallylow cure rates due to inefficient therapies as a result of theimpermeable specific characteristics of the blood brain barrier.Oxysterols, with amphiphilic properties and rapid exchange ratesbetween membranes are expected to cross easily the blood brainand the brain hematotumoral barriers being potentially useful as achemotherapeutic alternative for neuroblastoma and gliomatreatment. Murine oligodendrocytes (158N) cells with some char-acteristics of well differentiated oligodendrocytes [44], and mixedprimary culture of astrocytes and oligodendrocytes were used asmodel of noncancerous cells to take in consideration thepotential side effects on myelin synthesis (oligodendrocytes aremyelin synthetizing cells) and on cholesterol homeostasis, which istightly regulated in the neurons via tight connections with theastrocytes [45].
On transformed and tumoral cells (158N cells, C6 cells, SK-N-BE cells), under treatment with 7b-OHC and 7KC, as previouslyobserved on different cells of the vascular wall [13], the mostpotent effects, both on cell viability and cell growth, wereobserved with 7KC and 7b-OHC, and at a lower extend with 7a-OHC (Figs. 2e4AeC; Figs. 2e4FeH). Interestingly, 7a-OHC whichis not toxic on human monocytic leukemia cells (U937) [47] wascytotoxic on 158N and C6 cells but not on SK-N-BE cells. Previ-ously, 7b-OHC was also reported to induce cell death on thehuman neuroblastoma bone marrow derived cell line (SH-SY5Y)[25]. Noteworthy, under treatment with 4a-OHC and 4b-OHC, noeffect on cell viability, and more or less pronounced effects oncell growth were found on 158N and C6 cells in a range of con-centrations from 5 to 160 mM (Figs. 2e4 DE; Figs. 2e4 IJ), sup-porting a potential cytostatic activity of these molecules(Table 1). These later data are in agreement with those obtainedon HT-29 human colon carcinoma cells and ARPE-19 humanretinal pigmentary epithelial cells [24,25]. On mixed primaryculture of murine glial cells (astrocytes and oligodendrocytes), noeffects of 7a-OHC, 4a-OHC and 4b-OHC (used at 50 mM; in therange of the LD50 and IC50 of 7b-OHC) were revealed whateverthe cell type considered, whereas pronounced cytotoxic effectswere detected with 7b-OHC (marked decrease of cell viabilityand inhibition of cell growth), and at a lower extend with 7KC(Fig. 5A and B).
Overall, our data underline that the hydroxyl substrate in C7, onring B of the sterol nucleus, provides the highest cytotoxicity, evenon immortalized and normal glial cells calling thus into question itsselective cytotoxicity [25,48] (Table 1). They also show that hy-droxyl position at C4, on ring A of the sterol nucleus, has no effecton cell viability, and either more or less pronounced effects on cellgrowth of immortalized or tumoral cells (158N and C6 cells,respectively), or no effects on cell proliferation of normal glial cells(astrocytes, oligodendrocytes) (Table 1). Noteworthy, to date, 4b-OHC and 4a-OHC are the only oxysterols identified with cytostaticproperties suggesting that these molecules, whereas not cytotoxic,may have some interests to counteract cell proliferation. As 4b-OHCis a quantitatively dominating oxysterol in human circulation[10,11], it is suggested that it could be involved in the control of cellproliferation. In addition, as 4b-OHC has no cytotoxic effects,whatever the cell type considered, whereas it is a potent LXRa andLXRb agonist [29,30], this oxysterol or some of its derivatives mayhave some pharmacological interests to trigger LXRa and/or LXRbassociated metabolic pathways without interference on cellviability.
3. Conclusions
We described and validated a newmethodology for 4a-OHC and4b-OHC synthesis, and we bring new information establishing theabsence of effects of these oxysterols on cell viability as well asmore or less pronounced effects on cell growth (cytostasis) ofimmortalized and tumoral cells, and no effect on normal cells of theCNS. The cytostatic properties of 4a-OHC and 4b-OHC may havesome pharmacological interests.
4. Experimental methods
4.1. Materials and general methods
7-Ketocholesterol (7KC) was from SigmaeAldrich (St. Louis, MO,U.S.A.). The following oxysterols, 7b-hydroxycholesterol (7b-OHC)and 7a-hydroxycholesterol (7a-OHC) were either from SigmaeAldrich or a generous gift from Prof. M. Samadi (Département deChimie, LCP e A2MC, Université de Lorraine, Metz, France). The

Fig. 2. Effects of 7-ketocholesterol (7KC), 7-b-hydroxycholesterol (7b-OHC), 7a-hydroxycholesterol (7a-OHC), 4a-hydroxycholesterol (4a-OHC) and 4b-hydroxycholesterol (4b-OHC) on cell growth and viability of 158N murine oligodendrocytes cultured for 24 h in the absence or presence 7KC, 7b-OHC, 7a-OHC, 4a-OHC, and 4b-OHC in a range of con-centrations from 2.5 to 160 mM. Cell growth and viability were determined by cell counting in the presence of trypan blue. Data shown are mean � SD from three independentexperiments. Significance of the difference between untreated- and oxysterol-treated cells (ManneWhitney test; *P < 0.05 or less). No difference was observed between absolutecontrol and vehicle (ethanol: 0.05e0.32% corresponding to each oxysterol concentration).
T. Nury et al. / European Journal of Medicinal Chemistry 70 (2013) 558e567 561
purity of these oxysterols was determined to be 100% by gaseousphase chromatographyemass spectrometry.
All of the reactions leading to the synthesis of 4a-hydrox-ycholesterol (4a-OHC) and 4b-hydroxycholesterol (4b-OHC) werecarried out under an argon atmosphere. All reagents were ob-tained from commercial suppliers and used without further pu-rification. Flash chromatography was carried out using silica gel60 F254 (Merck) with mixtures of ethyl acetate and hexane aseluent unless9 specified otherwise. TLC analyses were performedon thin layer analytical Plates 60 F254 (Merck). Melting pointswere measured on a Reichert Kofler Thermopan apparatus andare uncorrected. 1H and 13C NMR spectra were recorded with a
Bruker Advance 250 spectrometer. High-resolution mass spectra(HRMS) were taken in electron ionization (EI) mode on JeolGCmate.
4.1.1. The general procedure for the synthesis of 1, 4, 6 and 7 was asfollows
To a solution of cholesterol or cholesterol acetate (3 mmol) inCHCl3 (30mL) was added SeO2 (4.2 mmol). The mixturewas heatedat reflux for 48 h for 3 and 24 h for 5. The reaction mixture was thencooled and activated carbon was added and then filtered off. Thesolvent was evaporated under reduced pressure and the residuewas purified over silica gel using EtOAcehexane as eluent.

Fig. 3. Effects of 7-ketocholesterol (7KC), 7-b-hydroxycholesterol (7b-OHC), 7a-hydroxycholesterol (7a-OHC), 4a-hydroxycholesterol (4a-OHC) and 4b-hydroxycholesterol (4b-OHC) on cell growth and viability of C6 rat glioma cells cultured for 24 h in the absence or presence 7KC, 7b-OHC, 7a-OHC, 4a-OHC, and 4b-OHC in a range of concentrations from2.5 to 160 mM. Cell growth and viability were determined by cell counting in the presence of trypan blue. Data shown are mean � SD from three independent experiments.Significance of the difference between untreated- and oxysterol-treated cells (ManneWhitney test; *P < 0.05 or less). No difference was observed between absolute control andvehicle (ethanol: 0.05e0.32% corresponding to each oxysterol concentration).
T. Nury et al. / European Journal of Medicinal Chemistry 70 (2013) 558e567562
4.1.2. Cholest-5-ene-3b,4b-diol (1) (also named 4b-hydroxycholesterol (4b-OHC))
Purified over silica gel using EtOAchexane (50e50). White solid(62% yield), mp 170e172 �C, IR (KBr): n: 3411, 2940, 1659 cm�1. 1HNMR (CDCl3): d 5.64 (dd, J ¼ 5.0, 2.0 Hz, 1H, 6-H), 4.1 (d, J ¼ 4.0 Hz,1H, 4a-H), 3.52 (m, 1H, 4-H), 1.18 (s, 3H, 19-CH3); 0.91 (d, J ¼ 6.5 Hz,3H, 21-CH3); 0.87 (d, J ¼ 6.5 Hz, 6H, 26-CH3 and 27-CH3); 0.68 (s,3H, 18-CH3) ppm. 13C NMR (CDCl3) d 142.9; 128.9; 77.4; 72.6; 57.0;56.2; 50.3; 42.4; 39.8; 39.6; 37.0; 36.3; 36.1; 35.9; 32.2; 31.9; 28.3;28.1; 25.5; 24.4; 23.9; 22.9; 22.7; 21.1; 20.6; 18.8; 12.0 ppm. HRMS(EI): m/z calcd for C27H46O2 [M] 402.3498, found 402.3489.
4.1.3. Cholest-5-ene-3b,7a-diol (4)Purified over silica gel using EtOAcehexane (70e30). White
solid (17% yield), mp 187e189 �C; IR (KBr): n: 3603, 3396, 2934,2867, 1663, 1467, 1383, 1056 cm�1. 1H NMR (CDCl3) d 5.5 (d,J ¼ 5.16 Hz, 1H, 6-H), 3.85 (broadt, 1H, 7b-H), 3.57 (m, J ¼ 11.0,5.4 Hz, 1H, 3-H), 0.99 (s, 3H, 19-CH3), 0.92 (d, 3H, J ¼ 6.4 Hz, 21-CH3), 0.86 (d, 6H, J ¼ 6.6 Hz, 26-CH3 and 27-CH3), 0.68 (s, 3H, 18-CH3) ppm. 13C NMR (CDCl3) d 146.2, 123.8, 71.2, 65.3, 55.8, 49.4,42.2, 42.1, 42.0, 39.5, 39.1, 37.5, 37.4, 37.0, 36.1, 35.7, 31.3, 28.2, 28.0,24.3, 23.7, 22.8, 22.6, 20.7, 18.7, 18.2, 11.60 ppm. HRMS (EI): m/zcalcd for C27H46O2 [M] 402.3498, found 402.3501.

Fig. 4. Effects of 7-ketocholesterol (7KC), 7-b-hydroxycholesterol (7b-OHC), 7a-hydroxycholesterol (7a-OHC), 4a-hydroxycholesterol (4a-OHC) and 4b-hydroxycholesterol (4b-OHC) on cell growth and viability of human neuroblastoma cells (SKeN-BE) cultured for 24 h in the absence or presence 7KC, 7b-OHC, 7a-OHC, 4a-OHC, and 4b-OHC in a range ofconcentrations from 2.5 to 160 mM. Cell growth and viability were determined by cell counting in the presence of trypan blue. Data shown are mean � SD from three independentexperiments. Significance of the difference between untreated- and oxysterol-treated cells (ManneWhitney test; *P < 0.05 or less). No difference was observed between absolutecontrol and vehicle (ethanol: 0.05e0.32% corresponding to each oxysterol concentration).
T. Nury et al. / European Journal of Medicinal Chemistry 70 (2013) 558e567 563
4.1.4. 3b-Acetoxy-4b-hydroxy-5-cholestene (6)Purified over silica gel using EtOAcehexane (20e80). White
solid (50% yield), mp 175e176 �C. IR (KBr): n: 3527, 3466, 2954,1740, 1714 cm�1. 1H NMR (CDCl3): d 5.62 (d, J ¼ 4 Hz, 1H, H-6),4.65 (td, J ¼ 12.6, 4.2. 3.6 Hz, 3-H), 4.17 (d, J ¼ 3 Hz, 1H, 4a-H),2.03 (s, 3H, OCOCH3), 1.15 (s, 3H, 19-CH3), 0.84 (d, J ¼ 6.5 Hz, 3H,21-CH3), 0.79 (d, J ¼ 6.5 Hz, 6H, 26-CH3 and 27-CH3), 0.61 (s, 3H,18-CH3) ppm. 13C NMR (CDCl3): d 170.1, 141.5, 129.4, 75.6, 75.4,56.8, 56.1, 56, 50.2, 42.2, 39.6, 39.5, 36.9, 36.16, 36.14, 32, 31.7,28.1, 27.9, 24.2, 23.8, 22.8, 22.5, 21.6, 21.3, 21, 20.4, 18.6, 11.8 ppm.HRMS (EI): m/z calcd for C29H48O3 [M] 444.3604, found444.3607.
4.1.5. 3b-Acetoxy-6a-hydroxy-4-cholestene (7)Purified over silica gel using EtOAcehexane (30e70). White
solid (25% yield), mp 140e142 �C. IR (KBr): n: 3457, 2936, 1742,1713, 1377, 1263 cm�1. 1H NMR (CDCl3): d 5.83 (m, J ¼ 5.2, 4.8,3.2 Hz, 1H, 3-H), 5.38 (dd, J ¼ 3.2, 0.8 Hz, 1H, 4-H), 3.6 (m,J ¼ 12.0, 4.8 Hz, 1H, 6b-H), 2.08 (s, 3H, OCOCH3), 1.18 (s, 3H, 19-CH3), 0.91 (d, J ¼ 6.6 Hz 3H, 21-CH3), 0.87 (d, J ¼ 6.6 Hz 6H, 26-CH3 and 27-CH3), 2.08 (s, 1H), 0.68 (s, 3H, 18-CH3) ppm. 13C NMR(CDCl3): d 171.2, 138.7, 131.5, 79.2, 71.7, 56.7, 56, 501, 42.2, 39.6,39.4, 36.8, 36.1, 35.9, 35.7, 32, 31.6, 28.1, 28, 25.7, 24.2, 23.8, 22.8,22.5, 21.6, 20.5, 20.3, 18.6, 11.8 ppm. HRMS (EI): m/z calcd forC29H46O2 [M � 18] 426.3498, found 426.3497.

Table 1Effects of oxysterols oxidized at C7 and C4 on cell viability and cell growth of different cell types of the central nervous system.
T. Nury et al. / European Journal of Medicinal Chemistry 70 (2013) 558e567564

Fig. 5. Effects of 7-ketocholesterol (7KC), 7-b-hydroxycholesterol (7b-OHC), 7a-hydroxycholesterol (7a-OHC), 4a-hydroxycholesterol (4a-OHC) and 4b-hydroxycholesterol (4b-OHC) on cell growth and viability of mixed murine primary culture of astrocytes and oligodendrocytes. After 10 days of culture, murine astrocytes/oligodendrocytes primary cellswere cultured for 24 h in the absence or presence 7KC, 7b-OHC, 7a-OHC, 4a-OHC, and 4b-OHC used at 50 mM. Cell growth and viability were determined by cell counting in thepresence of trypan blue. Data shown are mean � SD from three independent experiments. Significance of the difference between untreated- and oxysterol-treated cells (ManneWhitney test; *P < 0.05 or less). No difference was observed between absolute control and vehicle (ethanol: 0.1%).
T. Nury et al. / European Journal of Medicinal Chemistry 70 (2013) 558e567 565
4.1.6. 3b-Acetoxy-cholest-5-en-4-one (8)TPAP (17.6 mg, 0.05 mmol) was added to a solution of alcohol 5
(1 mmol, 444 mg), NMO (1.5 mmol, 175 mg), and activated Mo-lecular sieves, 4 �A (200 mg) in CH2Cl2 (5 mL). The mixture wasstirred for 10 h at rt under argon, filtered, concentrated, and puri-fied over silica gel using EtOAcehexane (10e90) as eluent to yieldthe keto compound 8 (398 mg, 90%) as a white solid. mp 118e120 �C, IR (KBr): n: 2953, 2867, 1753, 1705, 1692, 1625, 1373,1225 cm�1 1H NMR (CDCl3): d 6.35 (dd, J ¼ 2.5, 4.7 Hz 1H, 6-H), 5.19(dd, J ¼ 7.2, 12.0 Hz, 1H, 3-H), 2.1 (s, 3H, OCOCH3), 0.97 (s, 3H, 19-CH3), 0.9 (d, J ¼ 6.5 Hz, 3H, 21-CH3), 0.84 (d, J ¼ 6.5 Hz, 6H, 26-CH3 and 27-CH3), 0.67 (s, 3H, 18-CH3) ppm. 13C NMR (CDCl3)d 197.7, 170.1, 144.6, 134.1, 76.1, 56.3, 56, 49.1, 42.3, 39.5, 39,4, 39.1,36.1, 35.7, 34.5, 31.6, 31, 29.6, 28.1, 27.9, 25.6, 24.1, 23.7, 22.5, 21.3,21.1, 20.8, 18.6, 11.9 ppm. HRMS (EI): m/z calcd for C29H46O3 [M]442.3447, found 442.3401. Anal. Calc. for C29H46O3: C 78.12, H 10.63.Found. C 78.48, H 10.47.
4.1.7. Cholest-5-ene-3b,4a-diol (2)To solution of compound 8 (133 mg, 0.3 mmol) in THFeMeOH
(1:1) 5 mL, was added NaBH4 (23 mg, 0.6 mmol) at 0 �C and themixture was stirred at this temperature for 30 min. After 30 min
stirring at room temperature K2CO3 (83 mg, 0.6 mmol) was addedand the mixture was stirred over night. The solvent was evaporatedunder reduced pressure and the residue was dissolved in CH2Cl2and the organic layer was washed with water, brine dried overNa2SO4 and the solvent was evaporated under reduced pressure.The residue was purified over silica gel using EtOAcehexane (40e60) to give compound 8 96 mg (80%) as a white solid. mp 220e222 �C, IR (KBr): n: 3378, 2937, 1670, 1466, 1383 cm�1. 1H NMR(CDCl3): d 55.53 (dd, J ¼ 5.0, 2.0 Hz, 1H, 6-H), 3.77 (dd, J ¼ 8.0,2.0 Hz, 1H, 4b-H), 3.50 (m, 1H, 3-H), 0.95 (s, 3H, 19-CH3), 0.84 (d,J ¼ 6.4 Hz, 3H, 21-CH3), 0.79 (d, J ¼ 6.6 Hz, 6H, 26-CH3 and 27-CH3),0.62 (s, 3H,18-CH3) ppm. 13C NMR (CDCl3) d 141.9, 117, 76.5, 75, 56.7,56.12, 50.4, 42.2, 39.7, 39.5, 38, 36.6, 36.1, 35.7, 31.5, 31.3, 28.2, 28,27.9, 24.2, 23.8, 22.8, 22.5, 20.8, 20.2, 18.7, 11.8 ppm. HRMS (EI):m/zcalcd for C27H46O2 [M] 402.3498, found 402.3470.
1H and 13C NMR spectra of compounds 1, 2, 4, 6, 7 and 8 aregiven as Supplementary data.
4.2. Cell cultures and treatments
Murine oligodendrocytes (158N), with some characteristics ofdifferentiated oligodendrocytes [44], were immortalized with the

T. Nury et al. / European Journal of Medicinal Chemistry 70 (2013) 558e567566
SV40 large T-antigen derived from Tabby male (Ta/Y) control mice[32,33]. They were seeded at 120,000 cells per well in 12 wellsmicroplates containing 1 mL of culture medium, constituted byDulbecco’s modified Eagle medium (DMEM) (Lonza) supplementedwith 5% (v/v) heat-inactivated fetal bovine serum (FBS) (PanBiotech) and 1% antibiotics (100 U/mL penicillin, 100 mg/mLstreptomycin) (Pan Biotech).
Rat glioma cells (C6) were seeded at 250,000 cells per well in 12wells microplates containing 1 mL of culture medium, constitutedby Dulbecco’s modified Eagle medium F12 (DMEM F12) (Lonza)supplemented with 10% (v/v) heat-inactivated fetal bovine serum(FBS) (Pan Biotech) and 1% antibiotics (100 U/mL penicillin,100mg/mL streptomycin) (Pan Biotech).
Human neuroblastoma cells (SK-N-BE) were seeded at 400,000cells per well in 12 wells microplates containing 1 mL of culturemedium, constituted by Dulbecco’s modified Eagle medium(DMEM) (Lonza) supplemented with 10% (v/v) heat-inactivatedfetal bovine serum (FBS) (Pan Biotech) and 1% antibiotics (100 U/mL penicillin, 100 mg/mL streptomycin) (Pan Biotech).
158N, C6, and SK-N-BE cells were incubated at 37 �C in a hu-midified atmosphere containing 5% CO2, and passaged twice aweek. At each passage, cells were trypsinized with a (0.05% trypsin-0.02% EDTA) solution (Pan Biotech).
The conditions of treatment of murine oligodendrocytes 158Ncells, C6 rat glioma cells, and SK-N-BE human neuroblastoma cellswith the different oxysterols (4b-OHC, 7KC, 7b-OHC, 7a-OHC) wererealized as previously defined [49,50]. Initial concentrations ofoxysterols (800 mg/mL ¼ 2 mM) were prepared as follows: 1 mgoxysterolþ 50 mL absolute ethanolþ 1.2mL culturemedium. As 4a-OHC was more difficult to solubilize than the other oxysterols used,initial concentration of 4a-OHC (400 mg/mL ¼ 1 mM) was preparedas follows: 1 mg oxysterol þ 100 mL absolute ethanol þ 2.4 mLculture medium. Briefly, after plating 158N cells, C6 cells, and SK-N-BE cells for 24 h, cells were further treated for 24 h with variousconcentrations of these compounds (1e64 mg/mL) correspondingto (2.5e160 mM).
Mixed primary cultures of murine astrocytes and oligodendro-cytes, and conditions of treatments were performed as follows [30].One to two-days-old neonatal BALB/cJRj mice pups (Janvier-Europe) were washed with 70% ethanol and quickly beheaded.Briefly, the brains were removed and placed in a Petri dish con-taining 10 mL DMEM medium. Meninges and blood vessels wereremoved by rolling on a sterilized filter paper. The cleaned brainswere removed and placed in Petri dish containing 10 mL of freshmedium (DMEM/10% FCS/1% antibiotics). Brain tissue was gentlydiced into small pieces and crushed on a 100 mm cellular sieve. Cellswere harvested in 10 mL of DMEM/10% FCS/1% antibiotics in a50 mL tube. These 10 mL were used to seed one 12 wells plate(800 mL/well). Primary cultures were incubated in wet atmosphereat 37 �C, 5% CO2. After 4 days of culture, the mediumwas renewed,and then changed twice a week.
After 10 days of culture, different treatments were realized.Mixed primary cultures of murine astrocytes and oligodendrocyteswere realized in 12 wells plate for 24 h in the absence or in thepresence of 4a-OHC, 4b-OHC, 7KC, 7b-OHC, 7a-OHC (50 mM).
4.3. Evaluation of cell viability and cell growth
After trypsinization, cells were centrifuged and resuspended inculture medium. The percentage of viable cells (cell viability% ¼ number of trypan blue negative cells/total number of cells) andcell growth (cell growth (% control) ¼ number of trypan bluenegative cells in the assay/number of trypan blue negative cells inthe control) were determined with trypan blue under an invertedphase contrast microscope Diaphot (Nikon). These parameters
were determined after 24 h of culture in the absence or in thepresence of 4a-OHC, 4b-OHC, 7KC, 7b-OHC, and 7a-OHC used in arange of concentrations from 2.5 mM to 160 mM. This range ofconcentrations was chosen in order to calculate with accuracy the‘lethal dose 50%’ (LD50) as well as the half maximal inhibitoryconcentration (IC50). The lethal dose 50% (LD50) corresponds to theamount of compound required to kill 50% of the test population,and the inhibitory concentration (IC50) corresponds to the con-centration required to reduce by 50% the number of viable cells[30]. In agreement with previous investigations [24,25,50], anoxysterol was considered as a cell death inducer when its LD50 waslower than 50 mM; it was considered as an inhibitor of cell prolif-erationwhen its IC50 was lower than 50 mM. Thus, an oxysterol wasconsidered cytotoxic when its LD50 was lower than 50 mM and/orwhen its IC50was lower than 50 mM; it was considered as cytostaticwhen the inhibition of cell proliferation was not associated with adecrease of cell viability.
4.4. Statistical analysis
Analyses were carried out with WinSTAT� for Microsoft� Excel(version 2012.1). Since the biological variables were not normallydistributed, nonparametric ManneWhitney test was used. P values<0.05 were considered significant in all tests.
Appendix A. Supplementary data
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.ejmech.2013.09.028.
References
[1] G.H. Williams, Heart Fail. Rev. 10 (2005) 7e13.[2] A. Crosignani, M. Zuin, M. Allocca, M. Del Puppo, Clin. Chim. Acta 412 (2011)
2037e2045.[3] W.L. Miller, H.S. Bose, J. Lipid Res. 52 (2011) 2111e2135.[4] W.L. Miller, R.J. Auchus, Endocr. Rev. 32 (2011) 81e151.[5] C.F. Zorumski, S.M. Paul, Y. Izumi, D.F. Covey, S. Mennerick, Neurosci. Bio-
behav. Rev. 37 (2013) 109e122.[6] G.J. Schroepfer, Physiol. Rev. 80 (2000) 361e554.[7] L. Iuliano, Chem. Phys. Lipids 164 (2011) 457e468.[8] A. Vejux, M. Samadi, G. Lizard, J. Ophthalmol. 2011 (2011) 471947.[9] A. Otaegui-Arrazola, M. Menendez-Carreno, D. Ansorena, I. Astiasaran, Food
Chem. Toxicol. 48 (2010) 3289e3303.[10] O. Breuer, J. Lipid Res. 36 (1995) 2275e2281.[11] S.Dzeletovic, O. Breuer, E. Lund,U.Diczfalusy, Anal. Biochem.225 (1995) 73e80.[12] A. Vejux, L. Malvitte, G. Lizard, Braz. J. Med. Biol. Res. 41 (2008) 545e556.[13] A. Vejux, G. Lizard, Mol. Aspects Med. 30 (2009) 153e170.[14] J.R. Dwyer, N. Sever, M. Carlson, S.F. Nelson, P.A. Beachy, F. Parhami, J. Biol.
Chem. 282 (2007) 8959e8968.[15] S. Uno, K. Endo, Y. Jeong, K. Kawana, H. Miyachi, Y. Hashimoto, M. Makishima,
Biochem. Pharmacol. 77 (2009) 186e195.[16] K. Ragot, D. Delmas, A. Athias, T. Nury, M. Baarine, G. Lizard, Chem. Phys.
Lipids 164 (2011) 469e478.[17] S. Lemaire-Ewing, L. Lagrost, D. Néel, Atherosclerosis 221 (2012) 303e310.[18] K. Ragot, J.J. Mackrill, A. Zarrouk, T. Nury, V. Aires, A. Jacquin, A. Athias,
J.P. Barros, A. Véjux, J.M. Riedinger, D. Delmas, G. Lizard, Biochem. Pharmacol.(2013) pii: S0006-2952(13) 00151-2.
[19] S. Roussi, F. Gossé, D. Aoudé-Werner, X. Zhang, E. Marchioni, P. Geoffroy,M. Miesch, F. Raul, Apoptosis 12 (2007) 87e96.
[20] L. Clarion, M. Schindler, J. de Weille, K. Lolmède, A. Laroche-Clary, E. Uro-Coste, J. Robert, M. Mersel, N. Bakalara, Biochem. Pharmacol. 83 (2012) 37e46.
[21] Y.H. Ji, C. Moog, J.P. Beck, P. Bischoff, B. Luu, Cancer Biochem. Biophys. 11(1990) 45e57.
[22] J.W. Hyun, D. Weltin, V. Holl, J. Marchal, P. Dufour, B. Luu, P. Bischoff, Anti-cancer Res. 17 (1997) 2621e2626.
[23] C. Rakotoarivelo, M. Adamczyk, M. Desgeorges, K. Langley, J.G. Lorentz,A. Mann, D. Ricard, E. Scherrer, A. Privat, M. Mersel, Anticancer Res. 26 (2006)2053e2062.
[24] J.F. Carvalho, M.M. Silva, J.N. Moreira, S. Simões, M.L. Sá e Melo, J. Med. Chem.53 (2010) 7632e7638.
[25] J.F. Carvalho, M.M. Silva, J.N. Moreira, S. Simões, M.L. Sá E Melo, J. Med. Chem.54 (2011) 6375e6393.
[26] K. Wide, H. Larsson, L. Bertilsson, U. Diczfalusy, Br. J. Clin. Pharmacol. 65(2008) 708e715.

T. Nury et al. / European Journal of Medicinal Chemistry 70 (2013) 558e567 567
[27] U. Diczfalusy, H. Nylén, P. Elander, L. Bertilsson, Br. J. Clin. Pharmacol. 71(2011) 183e189.
[28] K. Bodin, L. Bretillon, Y. Aden, L. Bertilsson, U. Broomé, C. Einarsson,U. Diczfalusy, J. Biol. Chem. 276 (2001) 38685e38689.
[29] B.A. Janowski, P.J. Willy, T.R. Devi, J.R. Falck, D.J. Mangelsdorf, Nature 383(1996) 728e731.
[30] T. Nury, M. Samadi, A. Varin, T. Lopez, A. Zarrouk, M. Boumhras, J.M. Riedinger,D. Masson, A. Vejux, G. Lizard, Biochimie 95 (2013) 518e530.
[31] S. Ducheix, J.M. Lobaccaro, P.G. Martin, H. Guillou, Chem. Phys. Lipids 164(2011) 500e514.
[32] A.C. Feutz, D. Pham-Dinh, B. Allinquant, M. Miehe, M.S. Ghandour, Glia 34(2001) 241e252.
[33] M.S. Ghandour, A.C. Feutz, W. Jalabi, O. Taleb, D. Bessert, M. Cypher, L. Carlock,R.P. Skoff, Glia 40 (2002) 300e311.
[34] J. de Weille, C. Fabre, N. Bakalara, Biochem. Pharmacol. (2013) pii: S0006-2952(13) 00152-4.
[35] O. Rosenheim, W.W. Starling, J. Chem. Soc. (1937) 377e384.[36] E. Ma, T. Choi, Bull. Korean Chem. Soc. 30 (2009) 245e248.[37] P. Ghosh, J. Das, A. Sarkar, S.W. Ng, E.R.T. Tiekink, Tetrahedron 68 (2012)
6485e6491.
[38] S. Li, J. Pang, W.K. Wilson, J. Schroepfer, Chem. Phys. Lipids 99 (1999) 33e71.[39] R.E. Marker, O. Kamm, E.L. Wittle, J. Am. Chem. Soc. 60 (1938) 1071e1073.[40] R.E. Marker, E. Rohrmann, J. Am. Chem. Soc. 60 (1938) 1073e1075.[41] R.E. Marker, E. Rohrmann, J. Am. Chem. Soc. 61 (1939) 3022e3024.[42] S.V. Ley, J. Norman, W.P. Griffith, S.P. Mardsen, Synthesis (1994) 639e666.[43] L.F. Fieser, R. Stevenson, J. Am. Chem. Soc. 76 (1954) 1728e1733.[44] M. Baarine, K. Ragot, E.C. Genin, H. El Hajj, D. Trompier, P. Andreoletti,
M.S. Ghandour, F. Menetrier, M. Cherkaoui-Malki, S. Savary, G. Lizard,J. Neurochem. 111 (2009) 119e131.
[45] V. Leoni, C. Caccia, Biochem. Pharmacol. (2013) pii: S0006-2952(13) 00196-2.[46] J. Li, T.D. Thompson, J.W. Miller, L.A. Pollack, S.L. Stewart, Pediatrics 121 (2008)
e1470ee1477.[47] C. Miguet, S. Monier, A. Bettaieb, A. Athias, G. Besséde, A. Laubriet, S. Lemaire,
D. Néel, P. Gambert, G. Lizard, Cell Death Differ. 8 (2001) 83e99.[48] A. Kupferberg, G. Teller, P. Behr, C. Leray, P.F. Urban, G. Vincendon, M. Mersel,
Biochim. Biophys. Acta 1013 (1989) 231e238.[49] G. Lizard, S. Monier, C. Cordelet, L. Gesquière, V. Deckert, S. Gueldry, L. Lagrost,
P. Gambert, Arterioscler. Thromb. Vasc. Biol. 19 (1999) 1190e1200.[50] G. Lizard, V. Deckert, L. Dubrez, M. Moisant, P. Gambert, L. Lagrost, Am. J.
Pathol. 148 (1996) 1625e1638.