Immunohistochemistry Antibody Customer Review for Anti-IFN-γRα Polyclonal Antibody (STJ93645)
-
Upload
st-johns-laboratory-ltd -
Category
Science
-
view
83 -
download
1
Transcript of Immunohistochemistry Antibody Customer Review for Anti-IFN-γRα Polyclonal Antibody (STJ93645)

Result
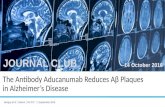
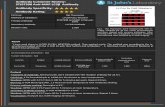
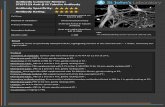
“Cleaner more specific staining at 1:100 and 1:300.” - B. Flood, Trinity College.
Cell Line: FFPE mouse distal colon tissue
Method of validation: Immunohistochemistry
Secondary Antibody Cell Signalling goat Anti-rabbit IgG, HRP-linked Antibody Cell Signalling (Cat. No: 7074S)
Dilution ratio: 1:100, 1:300
Protocol
Treatment of materials: FFPE AOM/DSS treated C57BL/6 distal colon tissue sectioned (5 μM) and mounted on glass slides.
Deparaffinise slides: Heat samples to 60°C for 30 minutes. Deparaffinise the tissues in Histoclear x2 (National Diagnos-tics) for 5 minutes in each. Rehydrate the slides in decreasing concentrations of ethanol (100%, 90% and 70%) for 5 minutes in each one. Then Incubate the slides in water/PBS for 5 minutes.
Antigen retrieval: Incubate the slides in boiling TRIS-EDTA buffer (pH 9.0) for 20 minutes in a microwave and then leave for cooling in the buffer for 25 minutes. Wash the slides 3 times for 5 minutes each in dH20.
Blocking endogenous peroxidase activity: Place slides in 3% H202 (1:10 dilution of 30% H202 in dH20) for 10 minutes. Wash the slides twice for 5 minutes each time in dH20. Then wash the slides in wash buffer for 5 minutes (1xPBS, 0.05% Tween). Do not allow the slides dry out.
Blocking: Place slides in incubation chamber with moist tissue underneath the pipettes. Blocking non-specific bindings: 5% BSA in 1x PBS. Apply with Pasteur pipette. Then apply slide covers ensuring no bubbles form. Cover incubation chamber with tinfoil covered lid and incubate for 40 minutes. Wash the slides in wash buffer for 5 minutes (1xPBS, 0.05% Tween).
Primary antibody: Place slides in incubation chamber with moist tissue underneath the pipettes. Apply 100ul of the specific primary antibody (IFN-γRα: 1:100 and 1:300 diluted in PBS containing 1% BSA ). Negative control: apply 100ul of PBS containing 1% BSA. Positive control: PCNA 1:100 diluted in PBS containing 1% BSA. Cover incubation chamber with tinfoil covered lid and incubate overnight at 4°C (cold room). Then wash the sections in PBS containing 0.05% Tween 20 (3-4 x5 minute washes on a shaker).
Secondary antibody: Place slides in incubation chamber with moist tissue underneath the pipettes. Apply 100ul appro-priate HRP secondary rabbit HRP (1:100). Add secondary Ab to each slide including negative control. Cover incubation chamber with tinfoil covered lid and incubate for 1 hour at 4°C (cold room). Wash 3 times for 5 minutes each in PBS/0.05% Tween.
DAB staining: Apply DAB substrate from BRDU Kit (1 drop of chromagen to 1 ml DAB buffer). Incubate for 5-10 minutes. Wash with PBS/0.05% Tween for 5 minutes and then place under running water for 5 minutes.
Counterstaining: Immerse in Sigma Haematoxylin (filtered and diluted 1:6 in dH2O) for 30-40 seconds exactly. Wash under running water for 5 minutes.
Dehydration and mounting: Dehydrate the slides in increasing concentrations of ethanol (70%, 90% and 100%) for 5 minutes in each one. Clear in xylene (3 X 5 minutes). Clean and dry each slide without touching tissue. Add a few drops of pertex or PDX onto the coverslip then gently lie the slide onto it. Gently press down on it with tip to spread mounting medium if necessary.
Antibody Customer Review: STJ93645 Anti-IFN-γRα antibody
Antibody Specificity:
Antibody Rating: