Family-wide Investigation of PDZ Domain-Mediated Protein-Protein Interactions Implicates β-Catenin...
Transcript of Family-wide Investigation of PDZ Domain-Mediated Protein-Protein Interactions Implicates β-Catenin...

Chemistry & Biology
Article
Family-wide Investigation of PDZ Domain-MediatedProtein-Protein Interactions Implicates b-Cateninin Maintaining the Integrity of Tight JunctionsTaranjit S. Gujral,1,2,3 Ethan S. Karp,2,3 Marina Chan,1 Bryan H. Chang,2 and Gavin MacBeath1,2,*1Department of Systems Biology, Harvard Medical School, Boston, MA 02115, USA2Department of Chemistry and Chemical Biology, Harvard University, Cambridge, MA 02138, USA3These authors contributed equally to this work*Correspondence: [email protected]
http://dx.doi.org/10.1016/j.chembiol.2013.04.021
SUMMARY
b-catenin is amultifunctional protein that plays a crit-ical role in cell-cell contacts and signal transduction.b-catenin has previously been shown to interact withPDZ-domain-containing proteins through its C termi-nus. Using protein microarrays comprising 206mouse PDZ domains, we identified 26 PDZ-domain-mediated interactions with b-catenin andconfirmed them biochemically and in cellular lysates.Many of the previously unreported interactionsinvolved proteins with annotated roles in tight junc-tions. We found that four tight-junction-associatedPDZ proteins—Scrib, Magi-1, Pard3, and ZO-3—colocalize with b-catenin at the plasma membrane.Disrupting these interactions by RNA interference,overexpression of PDZ domains, or overexpressionof the b-catenin C terminus altered localization ofthe full-length proteins, weakened tight junctions,and decreased cellular adhesion. These results sug-gest that b-catenin serves as a scaffold to establishthe location and function of tight-junction-associ-ated proteins.
INTRODUCTION
b-catenin is a ubiquitously expressed, multifunctional protein
that plays important roles in cell adhesion and signal transduc-
tion (Morin, 1999). It consists of an amino-terminal region con-
taining 12 armadillo repeats and a long carboxy-terminal tail. It
is frequently found at the plasma membrane, where it interacts
with E-cadherin to regulate cell adhesion, but it is also found in
the cytoplasm, where it plays a role in Wnt signaling, and in the
nucleus, where it acts as a transcriptional activator (Kumar
Kundu et al., 2006). Aberrant expression and/or localization of
b-catenin have been implicated in a wide variety of cancers,
most notably colorectal cancer (Abbosh and Nephew, 2005;
Harris and Peifer, 2005; Herynk et al., 2003; Morin, 1999).
Although b-catenin interacts with E-cadherin, adenomatous
polyposis coli (APC), and axin via its armadillo repeats, it also in-
teracts with PDZ-domain-containing proteins. PDZ domains
816 Chemistry & Biology 20, 816–827, June 20, 2013 ª2013 Elsevier
mediate protein-protein interactions by binding the C termini of
their target proteins. It was initially found that the PDZ domain
of dishevelled homolog 1 (Dvl-1) recognizes the C-terminal tail
of b-catenin and that this interaction is required to induce nuclear
accumulation of b-catenin (Axelrod et al., 1998). Subsequently,
several other PDZ-domain-mediated interactions were reported.
For example, TIP-1, which features a single PDZ domain, binds
b-catenin and inhibits b-catenin-dependent transcription (Kana-
mori et al., 2003). Similarly, MAGI-1b, which features six PDZ
domains, complexes with b-catenin at the plasma membrane
during the formation of cell-cell junctions (Dobrosotskaya and
James, 2000).
PDZ domains constitute one of the largest families of protein
interaction modules found in nature. In mammals, they facilitate
a wide range of cellular processes, including protein trafficking,
neuronal signaling, the establishment of cell polarity, and nutrient
uptake in the gut (Nourry et al., 2003). PDZ-domain-containing
proteins often act as molecular scaffolds to colocalize their bind-
ing partners and facilitate signaling (Kim and Sheng, 2004). As
b-catenin is able to interact with a variety of PDZ domains and
as these interactions affect the localization and activity of b-cat-
enin, we asked if a broad and unbiased screen for additional PDZ
domains that recognize b-catenin could uncover previously
unrecognized biological roles for this protein. To this end, we
prepared protein microarrays comprising 206 mouse PDZ do-
mains and probed them with a fluorescently labeled peptide
derived from the C terminus of b-catenin. Our arrays highlighted
nine previously reported interactions, as well as 17 additional in-
teractions, all of which we confirmed and quantified using a so-
lution-phase florescence polarization (FP) assay. In addition, all
26 PDZ domains captured b-catenin from cellular lysates, and
many of the full-length proteins from which these domains
were derived coimmunoprecipitated with b-catenin upon co-
transfection of HEK293 cells.
Interestingly, a survey of gene ontology revealed that many
of the previously unreported interactions involved proteins
that have previously been implicated in tight junctions. By
overexpressing isolated PDZ domains, by overexpressing the
PDZ-binding motif at the carboxyl terminus of b-catenin, or by
knocking down b-catenin, we found that these interactions
play a causal role in forming and maintaining the overall strength
and integrity of tight junctions. In addition, they inhibit b-catenin-
mediated cell adhesion, proliferation, and migration. These ob-
servations are consistent with a model in which b-catenin plays
Ltd All rights reserved

0
40
80
120
160
0 25 50 75 100 125 150
MUPP1
[PDZ Domain] (μM)
0
40
80
120
160
200
0 25 50 75Fluo
resc
ence
pol
ariz
atio
n (m
P)
Scrib
[PDZ Domain] (μM)
0
40
80
120
160
0 25 50 75 100 125Fluo
resc
ence
pol
ariz
atio
n (m
P)
Magi-1
[PDZ Domain] (μM)
0
40
80
120
0 10 20 30 40 50 60
[PDZ Domain] (μM)
Pard-3
Fluo
resc
ence
pol
ariz
atio
n (m
P)
Fluo
resc
ence
pol
ariz
atio
n (m
P)
Magi-1
ScribPard-3
MUPP1
A
B
KD = 13 μMKD = 1 μM
KD = 100 μM KD = 49 μM
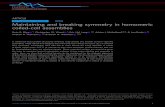
Figure 1. Identification of b-Catenin/PDZ-
Domain Interactions Using Protein Microar-
rays and Fluorescence Polarization
(A) Images of PDZ domain microarrays,
probed with a fluorescently labeled peptide
derived from the C terminus of b-catenin. The
Cy5 image (green) shows the placement of the
spots on one array and the 5(6)-TAMRA images
(red) show binding of the peptide to immobilized
PDZ domains. The PDZ domains were spotted in
duplicate from top to bottom as shown. In-
teractions with PDZ domains derived fromMagi-1,
Scrib, Pard3, and Mupp1 are highlighted in white
boxes.
(B) Confirmation and quantification of b-catenin/
PDZ-domain interactions by fluorescence polari-
zation. Saturation binding curves are shown for
PDZ domains derived from Magi-1, Scrib, Pard3,
and Mupp1.
See also Figure S1 and Table S1.
Chemistry & Biology
b-Catenin Helps Maintain Tight Junctions
an integral role in maintaining cell-cell contacts, participating not
only in adherens junctions but also in tight junctions. Misregula-
tion of these functions affects adhesion, proliferation, andmigra-
tion, consistent with the established role of b-catenin in cellular
transformation.
RESULTS
Family-wide Discovery of PDZ Domains that Recognizeb-CateninWe have previously shown that protein microarrays can be
used to uncover novel interactions between PDZ domains and
synthetic peptides representing the C-terminal tails of proteins
(Stiffler et al., 2006, 2007). In order to obtain a broad and
unbiased view of PDZ-domain-mediated interactions with
b-catenin, we prepared protein microarrays comprising 206
distinct PDZ domains. We then synthesized a fluorescently
labeled peptide that includes the six C-terminal residues of
b-catenin and used this peptide to probe our PDZ domain
microarrays. Upon washing and scanning for fluorescence, our
arrays highlighted 26 distinct interactions (Figure 1A; Figure S1;
Table S1 available online). To our knowledge, only nine interac-
tions between PDZ domains and b-catenin have previously
been reported (Table 1). All nine of these interactions were
Chemistry & Biology 20, 816–827, June 20, 2013
observed on ourmicroarrays and 17 addi-
tional interactions were also uncovered
(Table 1).
As a secondary assay, we used FP
to retest all 26 domain-peptide inter-
actions (Figure 1B). To quantify binding
affinities, we held the fluorescent peptide
at a constant concentration (20 nM)
and introduced purified PDZ domains at
16 concentrations, ranging from low
nanomolar to high micromolar. In all 26
cases, saturation binding was observed.
The domains spanned a wide range of
affinities, with many previously unre-
ported interactions exhibiting moderate to high affinities (KD <
50 mM; Table 1).
Interactions between Full-Length ProteinsBuilding on these domain-peptide interactions, we next asked if
isolated PDZ domains could recognize full-length b-catenin in
the context of complex cellular lysates. Each of the His6-tagged
PDZ domains was incubated with lysate derived from HEK293
cells, which naturally express b-catenin. Upon recovery of the
domains by immobilized metal affinity chromatography (IMAC),
we found that endogenous b-catenin copurified with 24 of the
26 domains (Figure 2A). No b-catenin was recovered when the
PDZ domain was omitted from the pull-down or when noncog-
nate PDZ domains were used (PDZ domains that did not recog-
nize the b-catenin peptide on our microarrays; Figure 2A).
To investigate interactions between full-length b-catenin and
full-length PDZ proteins, we focused on eight proteins for which
full-length human complementary DNAs (cDNAs) were readily
available: Mals2, Grasp55, Tiam2, PDZk7, Mast1, Dvl3,
OMP25, and Scrib. The coding regions for these eight proteins
were subcloned into a mammalian expression vector that ap-
pends an amino-terminal hemagglutinin (HA) tag. HEK293 cells
were then cotransfected with vectors expressing the HA-tagged
proteins and Myc-tagged b-catenin (Figure 2B). PDZ proteins
ª2013 Elsevier Ltd All rights reserved 817

Table 1. Biophysical Interactions between b-Catenin and PDZ
Domains
Previously Reported Interactions KD (mM)
Unreported
Interactions KD (mM)
Tip1 (Kanamori et al., 2003) 0.3 Magi-3 1
Magi-2 (Nishimura et al., 2002) 0.6 Tiam2 1
Magi-1 (Dobrosotskaya and James,
2000)
1 ZO-3 4
Erbin (Ress and Moelling, 2008) 6 Shank3 9
NHERF-1 (Theisen et al., 2007) 20 Scrib 13
LIN7 (Perego et al., 2000) 25 PSD95 31
Dvl1 (Song and Gelmann, 2005) 79 GRASP55 38
Dvl3 (Song and Gelmann, 2005) 155 Mast1 39
Grip1 (Song and Gelmann, 2005) 369 MUPP1 49
PTP-BL 79
Pard3 100
PDZk7 103
Neurabin-1 176
PDZk3 207
Synip 276
PDZ11 333
SAP97 342
See also Tables S2 and S3.
Chemistry & Biology
b-Catenin Helps Maintain Tight Junctions
were immunoprecipitated using an anti-HA antibody. Empty
Myc-tagged vector served as a negative control. b-catenin has
previously been shown to interact with Dvl3 and Mals2 (Perego
et al., 2000; Song et al., 2000). Both of these interactions were
observed, as expected (Figure 2C). In addition, all six previously
unreported interactions were observed (Figure 2D). A lesser
nonspecific interaction was also observed in negative controls
of Mals2 and OMP25 immunoprecipitates (Figure 2D).
b-Catenin Interacts with Tight-Junction-AssociatedProteinsHaving established that the domain-peptide interactions we
identified on our microarrays are also observed using full-length
proteins, we asked if our microarray results could provide new
insight into the biological role of b-catenin. Using the DAVID inte-
grated data mining environment (Dennis et al., 2003) and the
Gene Ontology (GO) database (http://www.geneontology.org/),
we classified the proteins in Table 1 according to biological pro-
cess, molecular function, and cellular components associated
with each protein (Table S2). Not surprisingly, annotation of bio-
logical process and molecular function varied widely among the
nonhomologous PDZ domain-containing proteins. When all of
the cellular components associated with each PDZ protein
were grouped, however, we observed significant overrepresen-
tation of proteins associated with either tight junctions or their
neurological equivalent, the synaptosome (pz10�5) (Figure 3A).
It is well established that components of adherens junctions,
such as E-cadherin, a-catenin, and p120 catenin, play a role in
the formation of tight junctions (Itoh et al., 1997; Schneeberger
and Lynch, 2004). To date, however, it has only been speculated
that b-catenin is associated with tight junctions; how this occurs
818 Chemistry & Biology 20, 816–827, June 20, 2013 ª2013 Elsevier
has not yet been explored (Dobrosotskaya and James, 2000;
Shin et al., 2006).
To investigate this role for b-catenin, we chose four proteins
in Table 1 that have well-documented roles in the formation or
maintenance of tight junctions (Bilder and Perrimon, 2000; Tsu-
kita et al., 2001): one that has previously been shown to interact
with b-catenin (Magi-1) and three that have not (Pard3, ZO-3,
and Scrib). Each of these proteins features three or more PDZ
domains (Figure 3B). To establish which of these domains recog-
nize b-catenin, we transfected HEK293 cells with the coding re-
gions for Myc-tagged versions of each of the individual PDZ do-
mains as well as with several clusters of adjacent PDZ domains.
Upon immunoprecipitating each domain and immunoblotting for
endogenous b-catenin, we found that b-catenin was bound by
PDZ domains 4 and 5 of Magi-1; PDZ domain 3 of Pard3; PDZ
domains 1, 2, and 3 of Scrib; and PDZ domain 2 of ZO-3. PDZ
domain 6 of Magi-1 and PDZ domain 1 of Pard3 bound b-catenin
weakly (Figure 3C). All of the PDZ domain clusters that included
these cognate domains also bound b-catenin in the coimmuno-
precipitation assay (Figure 3C). Importantly, these biochemical
data are consistent with the biophysical data obtained from the
protein microarray and FP assays (Table 1).
b-Catenin Colocalizes with Tight-Junction-AssociatedProteinsAll four PDZ proteins—Magi-1, Pard3, ZO-3, and Scrib—as well
as b-catenin are endogenously expressed in Madin-Darby
canine kidney (MDCK) cells. Using confocal fluorescence micro-
scopy, we found that b-catenin is largely confined to the plasma
membrane and colocalizes with all four proteins (Figure 4A). In
SW480 colorectal cancer cells, however, b-catenin escapes
the APC/Axin degradation complex and accumulates in the
cytosol and nucleus (Nath et al., 2003; Smith et al., 1993). As ex-
pected, we observed strong localization of b-catenin in both the
cytosol and nucleus in these cells (Figure 4B). Interestingly, all
four tight-junction-associated PDZ proteins do not localize at
the plasma membrane in these cells but are instead found
primarily in the cytoplasm (Figure 4B). This suggests that locali-
zation of b-catenin may affect localization of its PDZ-domain-
containing binding partners. Consistent with this hypothesis,
we found that knocking down the levels of b-catenin in
HEK293 cells resulted in higher cytosolic levels of exogenously
expressed Scrib and Pard3 (Figure S2). Similarly, knocking
down the levels of b-catenin in Caco-2 epithelial cells resulted
in decreased membranous expression of endogenous Scrib
(Figure 4C). Further, consistent with previous studies and the
documented role of Scrib in tight junction assembly, knocking
down levels of Scrib reduced localization of Occludin, a marker
for tight junctions (Figure S3). Knocking down Scrib in MDCK
cells (2-fold), however, did not affect the membranous localiza-
tion of b-catenin, consistent with the notion that b-catenin drives
localization of its tight junction protein binding partners rather
than the other way around (Figure 4D).
Overexpressing Isolated PDZ Domains Affects TightJunctionsTo investigate the functional role of PDZ-domain-mediated inter-
actions in tight junction biology, we performed a series of exper-
iments in which we transfected MDCK cells with constructs
Ltd All rights reserved

IP: Myc
HA-Dvl3Myc-β-catenin
IB: HA
IB: Myc
Myc-β-cateninHA-Tiam2
IB: HA
IB: Myc
IP: Myc
IP: Myc
HA-OMP25Myc-β-catenin
IB: HA
IB: Myc
IP: Myc
IP: Myc
HA-Grasp55Myc-β-catenin
IB: HA
IB: Myc
IP: Myc
IP: Myc
HA-Pdzk7Myc-β-catenin
IB: HA
IB: Myc
IP: Myc
IP: Myc
HA-Mast1Myc-β-catenin
IB: HA
IB: Myc
IP: Myc
IP: Myc
IB: HA
IB: Myc
IP: Myc
IP: Myc
Myc-β-cateninHA-ScrbHA-Dvl3
HA-Grasp55HA-Mals2HA-Mast1HA-OMP25
HA-Pdzk7HA-Tiam2
Myc-β-catenin
HA-Scrb
IB: Myc
Lysates
Lysates
150 100
75
250
50
25
kDa
IB: HA
A
B D
C
+ + + + + + + + + -+ - - - - - - - - -- + - - - - - - - -- - + - - - - - - -- - - + - - - - - -- - - - - + - - - -- - - - + - - - - -- - - - - - + - - -- - - - - - - + - -
+ - + -+- + - Myc-β-catenin
HA-Mals2
IB: HA
IB: MycIP: Myc
+ - + -+- + -
+ - + -+- + -
+ - + -+- + -
+ - + -+- + -
+ - + -+- + -
+ - + -+- + -
+ - + -+- + -
PS
D95
(3/3
)
PD
Zk11
(1/1
)O
MP
25(1
/1)
Scr
ib(3
/4)
PTP
-BL(
2/5)
Mag
i-3(5
/5)
Grip
1(1/
7)M
agi-3
(3/5
)P
DZk
3(3/
6)D
vl1(
1/1)
Dvl
3(1/
1)N
eura
bin-
1(1/
1)
Mag
i-1(6
/6)
Mas
t1(1
/1)
Sha
nk3(
1/1)
Cha
psyn
-110
(3/3
)S
ynte
nin-
2(1/
2)H
is6-
Thio
redo
xin
Syn
ip(1
/1)
PD
Zk7(
1/1)
Gra
sp55
(1/1
)Ti
am2(
1/1)
SA
P(9
7(1,
2,3/
3)M
UP
P!(1
,2,3
/13)
NH
ER
F1(1
,2/2
)P
ard3
(1,2
,3/3
)ZO
-3(1
,2,3
/3)
Cha
psyn
-110
(3/3
)S
ynte
nin-
2(1/
2)H
is6-
Thio
redo
xin
IB:β-catenin
FP Pos
itive
FP Pos
itive
FP Neg
ative
FP Neg
ative
7878
100
IP: Myc IP: Myc
7510075
10075
10075
10075
10075
10075
10075
250 250
10025
5025
50150
Figure 2. Validation of b-Catenin/PDZ-Domain Interactions by His6 Pull-Down and Co-IP Assays
(A) Western blots showing that full-length b-catenin fromHEK293 cells binds to purified PDZ domains in a His6 pull-down assay. Noncognate PDZ domains serve
as negative controls. The numbers in parentheses refer to the PDZ domain number (counting from the N terminus) and the total number of PDZ domains in the full-
length sequence. Data shown are representative images from two independent experiments.
(B) Western blot showing expression of full-length Myc-tagged b-catenin (top) and full-length HA-tagged PDZ-domain-containing proteins (bottom) in whole-cell
lysates derived from transiently transfected HEK293 cells. Data shown are representative images from three independent experiments.
(C and D) Full-length b-catenin associates with full-length HA-tagged PDZ-domain-containing proteins. HEK293 cells were transiently cotransfected with Myc-
tagged b-catenin and full-length HA-tagged PDZ-domain-containing proteins. Myc-tagged b-catenin was immunoprecipitated with an anti-Myc antibody and
copurifying proteins were detected by immunoblotting with an anti-HA antibody. (C) Previously reported interactions between b-catenin and both Dvl3 andMals2
were confirmed. (D) Previously unreported interactions were observed between b-catenin and Scrib, Tiam2, Grasp55, Omp25, Mast1, and Pdzk7. Data shown
are representative images from three independent experiments.
See also Figure S1.
Chemistry & Biology
b-Catenin Helps Maintain Tight Junctions
expressing isolated Myc-tagged PDZ domains derived from
Magi-1, Pard3, ZO-3, and Scrib. We reasoned that isolated
PDZdomains,whenoverexpressed,would competewith endog-
enous PDZ proteins for binding to b-catenin. They would there-
fore act as dominant negatives. We started by using confocal
microscopy to visualize the subcellular localization of occludin,
a tight junction marker protein (Furuse et al., 1993), as well as
the four PDZ proteins and b-catenin. In untransfected MDCK
cells, Scrib localized at the plasmamembrane (Figure 5A). In cells
transfected with Myc-tagged PDZ3 of Scrib, however, increased
levels of cytosolic Scrib were observed (Figure 5A). In addition,
only the cells expressing PDZ3 exhibited truncated and discon-
tinuous occludin staining at the cell borders (Figure 5A). Similar
results were observed when cognate PDZ domains from Magi-
1, Pard3, and ZO-3 were overexpressed (Figure S4). As a nega-
Chemistry & Biology 20,
tive control, we used PDZ4 of Scrib, which does not recognize
b-catenin. Overexpression of this domain had no appreciable
affect on the localization of either occludin or Scrib (Figure 5B).
Interestingly, none of these PDZ domains had an appreciable
effect on the localization of b-catenin itself, suggesting that the
localization of b-catenin is dominant and affects the localization
of its PDZ domain-containing binding partners.
One way in which the integrity of tight junctions can be quan-
tified is to measure the length of occludin in individual cells (Chen
and Macara, 2006; Wan et al., 1999). Longer continuous
stretches of occludin indicate well-formed tight junctions. To
quantify the effect of disrupting PDZ-protein/b-catenin interac-
tions on tight junction integrity, we measured the mean occludin
length in untransfected MDCK cells and in cells overexpressing
isolated PDZ domains. In every case, overexpressing cognate
816–827, June 20, 2013 ª2013 Elsevier Ltd All rights reserved 819

PDZ1PDZ2
PDZ3PDZ1
-2-3
Negati
ve
PDZ1PDZ2
PDZ3PDZ1
-2-3
Negati
ve
PDZ1PDZ2
PDZ3PDZ4
PDZ1-2
PDZ3-4
Negati
ve
PDZ1PDZ2
PDZ3PDZ4
PDZ6PDZ4
-5
Negati
ve
ZO-3 PDZ1
32-110 196-264 431-504
N C
Guanylate kinase-like
domainSH3PDZ2 PDZ3
ZO-3
Pard-3282-361 469-548 599-684
PDZ1 PDZ2 PDZ3N C
Pard-3
Scrib722-801 856-936 998-1079 1095-1178
PDZ1 PDZ2 PDZ3 PDZ4Leucine-rich
repeatsN C
Scrib
Magi-1
Magi-1
643-715 841-917 949-1065 1140-1214472-54826-105
WWPDZ1 PDZ2 PDZ3 PDZ4 PDZ5 PDZ6Guanylate kinase-like
domainN C
Adherens Junction
Synaptosome
Tight Junction Synapse
PostsynapticMembrane
Cytoskeleton/Cytoplasm
Cell-Cell Junction/Membrane
Endoplasmic Membrane
A B
C
Lysates
Lysates
IP: Myc
IP: Myc
IB: β-catenin
IB: Myc
IB: Myc
IB: β-cateninLysates
Lysates
IP: Myc
IP: Myc
IB: β-catenin
IB: Myc
IB: Myc
IB: β-catenin
Lysates
Lysates
IP: Myc
IP: Myc
IB: β-catenin
IB: Myc
IB: Myc
IB: β-catenin Lysates
Lysates
IP: Myc
IP: Myc
IB: β-catenin
IB: Myc
IB: Myc
IB: β-catenin
5625
7878
2516
56
1616
78
78
78 7878
78
16
56
16
25
16
56
16
16
25
Figure 3. b-Catenin Interacts with Several Tight-Junction-Associated PDZ-Domain-Containing Proteins
(A) Pie chart showing the cellular component classification as reported in the Gene Ontology database (http://www.geneontology.org) of all PDZ-domain-
containing proteins that were identified as binding partners of b-catenin. PDZ-domain-containing proteins that are known to localize in tight junctions are
highlighted in green.
(B) Domain structures of Magi-1, Pard3, Scrib, and ZO-3.
(C) Investigation of which PDZ domains in Magi-1, Pard3, Scrib, and ZO-3 bind full-length endogenous b-catenin in a co-IP assay. HEK293 cells were transiently
transfected with Myc-tagged versions of isolated PDZ domains or PDZ domain clusters derived from Magi-1, Pard3, Scrib, and ZO-3. PDZ domains were
immunoprecipitated using an anti-Myc antibody and interactions were detected by immunoblotting for b-catenin. Whole-cell lysates showing expression of the
Myc-tagged PDZ domains and endogenous b-catenin are also displayed. Data shown are representative images from at least three independent experiments.
Chemistry & Biology
b-Catenin Helps Maintain Tight Junctions
PDZ domains caused a significant decrease in the length of oc-
cludin per cell (p < 0.05; Figure 5C), whereas overexpressing the
noncognate PDZ4 of Scrib had no effect. The largest effect was
observed when PDZ6 of Magi-6 was overexpressed, consistent
with the observation that this is the highest affinity interaction
(KD = 1 mM).
Tight junction integrity can also be assessed by measuring
transepithelial electrical resistance (TEER). To assess the effect
of disrupting PDZ domain-mediated interactions with b-catenin,
we measured TEER in untransfected MDCK cells and in cells
transfected with isolated PDZ domains. As with the occludin
assay, overexpressing PDZ domains that recognized b-catenin
resulted in significant decreases in tight junction integrity (p <
0.05; Figure 5D), whereas overexpressing PDZ4 of Scrib had
no effect.
To delineate whether b-catenin plays a role in maintaining tight
junctions or forming them,we performed a calcium switch assay,
820 Chemistry & Biology 20, 816–827, June 20, 2013 ª2013 Elsevier
commonly used to monitor the development of tight junctions in
MDCK cells. In the Ca2+-switch assay, we maintained MDCK
monolayers in low calcium medium and subsequently trans-
ferred them to normal calcium-containing medium, which in-
creases cell-cell contacts and initiates assembly of junctional
complexes (Figure 5E). At selected times, cells were fixed and
the presence of tight junctions was monitored by visualizing
localization of occludin by immunofluorescence. Before the
Ca2+-switch (0 hr), the tight junction protein occludin showed a
discontinuous staining pattern in both parental MDCK cells
and in cells overexpressing isolated PDZ domains (Figures 5E
and 5F). In every case, overexpressing cognate PDZ domains
caused a significant decrease in the length of occludin per cell
(p < 0.05), whereas overexpressing the noncognate PDZ4 of
Scrib had no effect (Figure 5F).
b-catenin has been implicated in wide variety of cellular pro-
cesses, including cell adhesion, proliferation, and migration
Ltd All rights reserved

Figure 4. Localization of b-Catenin and Tight-Junction-Associated PDZ Proteins in Epithelial Cells
(A) Confocal images showing that b-catenin (red) colocalizes with the PDZ-domain-containing tight junction proteins Magi1, Pard3, Scrib, and ZO-3 (green) in
MDCK kidney epithelial cells. Scale bar is 10 mm.
(B) In SW480 colon cancer cells, b-catenin (red) is mostly localized in the cytoplasm and nucleus, whereas the PDZ domain-containing tight junction proteins
Magi1, Pard3, Scrib, and ZO-3 are localized in the cytoplasm. Themerged images showing both b-catenin and the PDZ-domain-containing tight junction proteins
are displayed to the right. Yellow indicates colocalization. Nuclei are stained in blue (Hoechst dye). Data shown are representative images from at least two
independent experiments. Scale bar is 10 mm.
(C) Confocal images showing b-catenin (red) colocalizes with the PDZ-domain-containing tight junction protein Scrib (green) in Caco-2 epithelial cells (top).
Knocking down the levels of b-catenin results in decreased membranous expression of endogenous Scrib (bottom).
(D) Knocking down the levels of Scrib in MDCK cells does not affect the membranous localization of b-catenin.
See also Figures S2 and S3.
Chemistry & Biology
b-Catenin Helps Maintain Tight Junctions
(Blankesteijn et al., 2000; Gavert and Ben-Ze’ev, 2007). To
further explore the functional relevance of our interactions, we
assessed the ability of MDCK cells expressing dominant nega-
tive PDZ domains to attach to extracellular matrix. Untransfected
MDCK cells adhere more strongly to Collagen I than to Fibro-
nectin or Laminin I (Figure 6A). Consistent with the loss of tight
junction integrity, MDCK cells expressing b-catenin-binding
PDZ domains exhibited a decreased ability to attach to all three
matrices (Figure 6A). When grown on Fibronectin, MDCK cells
overexpressing PDZ domains from Pard3, Scrib, and ZO-3
showed significantly less adhesion whereas MDCK cells overex-
pressing PDZ domains from Magi-1, Pard3, Scrib, and ZO-3
Chemistry & Biology 20,
all showed loss of adhesion on Collagen I. Overexpression of
PDZ domains from Magi-1, Pard3, and ZO-3 displayed loss of
adhesion on Laminin I, whereas MDCK cells expressing the non-
cognate PDZ4 of Scrib showed no change in their adhesion
properties relative to untransfected cells.
In a similar fashion, we also assessed the effect of expressing
isolated PDZ domains on cell proliferation using an MTT viability
assay and on cell migration using aBoyden chamber assay. Both
proliferation and migration increased when the dominant-
negative PDZ domains were overexpressed; no change was
observed when the control PDZ domain was overexpressed
(Figure 6B; Figure S5).
816–827, June 20, 2013 ª2013 Elsevier Ltd All rights reserved 821

Figure 5. Overexpression of b-Catenin-Interacting PDZ Domains Affects the Strength of Tight Junctions
(A) Confocal images showing membranous localization of endogenous Scrib MDCK cells (i) and cytosolic localization of Myc-tagged PDZ3 of Scrib (ii, v, and viii).
MDCK cells expressing PDZ3 of Scrib also showed altered expression of the tight junction marker occludin (iii and iv) and endogenous Scrib (vi and vii). The white
arrows indicate localization of occludin in cells that do not expressMyc-tagged PDZ3 of Scrib or PDZ6 ofMagi-1. The yellow arrows indicate occludin localization
in cells expressing PDZ3 of Scrib. Themembranous localization of endogenous b-catenin in cells expressing PDZ3 of Scrib is also shown (ix and x). Blue indicates
nuclei (Hoechst stain), and yellow indicates colocalization. Scale bars are 10 mm.
(B) MDCK cells expressing PDZ4 of Scrib (i, iv, and vii) do not show altered expression or localization of either occludin (ii and iii) or endogenous Scrib (v and vi).
The membranous localization of endogenous b-catenin in cells expressing PDZ4 of Scrib is also shown (viii and ix).
(legend continued on next page)
Chemistry & Biology
b-Catenin Helps Maintain Tight Junctions
822 Chemistry & Biology 20, 816–827, June 20, 2013 ª2013 Elsevier Ltd All rights reserved

Chemistry & Biology
b-Catenin Helps Maintain Tight Junctions
Overexpressing the PDZ Domain-Binding Motif at theCarboxyl Terminus of b-Catenin Affects Tight JunctionsAs PDZ domains are often able to recognize more that one pro-
tein, it is possible that overexpressing isolated PDZ domains also
affects interactions with proteins other than b-catenin. As the
converse of the experiments detailed in Figure 5, we sought to
perturb specific interactions between b-catenin and tight-junc-
tion-associated PDZ proteins by overexpressing GFP displaying
the carboxyl terminus of b-catenin (-GWFDTDL-CO2H) (Fig-
ure 6A). Our biochemical data show that this PDZ-domain-bind-
ing motif is capable of binding to tight-junction-associated PDZ
proteins (Figure 1). Thus, overexpressing this GFP construct
(GFP-bcat-Cterm) should act as a dominant negative by
competing with endogenous full-length b-catenin in binding to
tight-junction-associated PDZ proteins. We performed the
same series of experiments as described above, but this time
transfecting MDCK cells with GFP-bcat-Cterm or with GFP-
mut-Cterm, a nonbinding mutant version of GFP-bcat-Cterm in
which the last three residues were mutated to alanine (NH2-
GWFDAAA). In untransfected MDCK cells, endogenous Scrib
localized at the plasma membrane, as observed by confocal mi-
croscopy (Figure 6B). In cells transfected with GFP-bcat-Cterm,
however, increased levels of cytosolic Scrib were observed (Fig-
ure 6B). Further, GFP-bcat-Cterm and endogenous Scrib were
observed to colocalize in the cytosol, consistent with the hypoth-
esis that the C-terminal tail of b-catenin drives the localization of
Scrib (Figure 6B). In contrast, overexpressing GFP-mut-Cterm
had no appreciable affect on the subcellular localization of Scrib
and did not colocalize with endogenous Scrib (Figure 6B).
Next, we analyzed the de novo assembly of tight junctions using
the calcium switch assay, as described above. Overexpressing
GFP-bcat-Cterm caused a significant decrease in the average
length of occludin per cell, whereas overexpressing GFP-mut-
Ctermhadnoeffect (p<0.05; Figure6C).Consistentwith these re-
sults, transmission electron microscopy (TEM) images of MDCK
cells overexpressing GFP-mut-Cterm showed the presence of
typical tight junctions, as well as adherens junctions, in sites of
cell-cell contact (Figure 6D). In contrast, cells overexpressing
GFP-bcat-Cterm showedweaker or no tight junctions (Figure 6D).
Consistent with the loss of tight junction integrity, MDCK cells
expressing GFP-bcat-Cterm also exhibited a decreased ability
to attach to extracellular matrices (Figure 6E). When grown on
Fibronectin,Collagen I, or Fibrinogen,MDCKcells overexpressing
GFP-bcat-Cterm exhibited significantly less adhesion, whereas
MDCK cells expressing GFP-mut-Cterm showed little or no
(C) Quantification of occludin localization in parental MDCK cells or in cells expr
Occludin length was measured using ImageJ (http://rsbweb.nih.gov/ij/). Error ba
(D) Transepithelial electrical resistance (TEER), measured in parental MDCK cells a
least three biological replicates, error bars represent the SEM, and asterisks den
(E) A Ca2+-switch assay was performed with parental or MDCK cells expressing M
stained for occludin and Myc tag. Confocal images of occludin (green) and Myc
(F) Graphs show the results of quantification of occludin recruitment to cell-cell con
is the mean of at least three biological replicates, and error bars represent SEM.
(G) Relative adhesion of parental MDCK cells and cells expressing isolated PDZ
measured 48 hr posttransfection. Adhesion values are the means of at least thre
significant difference from mock-transfected parental cells (p < 0.05).
(H) Relative proliferation of parental MDCK cells and cells transfected with isolate
replicates, error bars represent the SEM, and asterisks denote a significant diffe
See also Figures S4 and S5.
Chemistry & Biology 20,
change in their adhesion properties relative to untransfected cells
(Figure 6E). Finally, overexpressing GFP-bcat-Cterm caused sig-
nificant (p < 0.05) increases in migration and invasion; no signifi-
cant change was observed with the mutant control (Figures 6F
and 6G). Taken together, these results show that PDZ-domain-
mediated interactionsbetweenb-cateninand tight-junction-asso-
ciated proteins affect a variety of b-catenin-mediated functions.
DISCUSSION
b-catenin is a central component of the cadherin cell adhesion
complex and plays an essential role in Wnt signaling. Previous
studies have shown that b-catenin is a physiological ligand of
PDZ domains. Here, we report a family-wide, unbiased examina-
tion of PDZ-domain-mediated interactions with b-catenin. Using
PDZ domain microarrays, we rediscovered all nine previously re-
ported b-catenin/PDZ-domain interactions and uncovered 17
additional interactions. All 24 PDZ domains were able to pull
down full-length b-catenin from cell lysates. In addition, when
we tested a representative sample of eight full-length PDZ
domain-containing proteins, all eight copurified with b-catenin
from cotransfected cells. It is therefore likely that most, if not
all, of the previously unreported interactions can occur in the
complex environment of the cell.
The full set of PDZ-domain-mediated interactions with b-cate-
nin can be visualized by constructing a b-catenin-centric
network diagram (Figure S6). This diagram includes all of the
PDZ proteins that were found to interact with b-catenin as well
as other proteins that have previously been identified as binding
partners for the PDZ proteins. This diagram offers further insight
into the biological role of b-catenin. For example, b-catenin has
previously been associated indirectly with PSD95 and Tiam2
through Lin7 and Src (Gottardi and Gumbiner, 2004; Piedra
et al., 2003). Here, we see that PDZ domains in PSD95 and
Tiam2 directly recognize b-catenin. Given the high connectivity
of this network, along with the fact that many of these proteins
are expressed in the same cells or tissues (Fanning and Ander-
son, 1999) (Table S3), it is likely that several different proteins
are interacting with b-catenin in the same cell at the same
time. One way in which these competing interactions could be
regulated is through subcellular localization of b-catenin. Some
PDZ proteins are confined to the cytoplasm, where they could
affect the signaling function of b-catenin, whereas others are
confined to the plasma membrane, where they could affect its
ability to stabilize cell-cell junctions.
essing isolated PDZ domains derived from tight-junction-associated proteins.
rs indicate the SEM of at least four measurements.
nd in cells expressing individual PDZ domains. TEER values are the mean of at
ote a significant difference from mock-transfected parental cells (p < 0.05).
yc-Scrib (PDZ3) and Myc-Scrib (PDZ4) constructs. Cell layers were fixed and
tag-scrib PDZ domain (red) are shown.
tacts in parental or MDCK cells overexpressing isolated PDZ domains. The bar
domains grown on Fibronectin, Collagen I, and Laminin I. Cell adhesion was
e biological replicates, error bars represent the SEM, and asterisks denote a
d PDZ domains. Each proliferation value is the mean of at least three biological
rence from mock-transfected parental cells (p < 0.05).
816–827, June 20, 2013 ª2013 Elsevier Ltd All rights reserved 823

Rel
ativ
e m
igra
tio (A
.U)
1.6
1.2
0.8
0.4
0.0
control
2.4
2.0
0.0
0.4
0.8
1.2
1.6
Rel
ativ
e in
vasi
on (A
.U)
Rel
ativ
e ad
hesi
on (A
.U) 1.0
0.8
0.6
0.4
0.2
0.0
Fibron
ectin
Collag
en I
Fibrino
gen
*
*
*
*
GFPN WFDTDL CPDZ binding
motif
GFPN WFDAAA C
GFP-β-cat-Cterm
GFP-mut-CtermPDZ binding
motif
Scrib GFP Merge
β-ca
teni
nc-
term
β-
cate
nin
mut
c-te
rm
Colocolization
50
100
150
300
0
Occ
ludi
n le
ngth
/cel
l (pi
xels
)
250
200
1hr Ca2+ 2hr Ca2+
GFP-β-cat-CtermGFP-mut-Cterm
0hr Ca2+
** **
parental
GFP-β-cat-Cterm GFP-β-cat-CtermGFP-mut-Cterm
GFP-β-cat-CtermGFP-mut-Cterm
A B
D
F G
C
E
Figure 6. Overexpressing the PDZ-Domain-Binding Motif of b-Catenin Affects Tight Junctions
(A) Schematic showing the carboxyl terminus of b-catenin (GFP-bcat-Cterm: NH2-GWFDTDL-COOH) andGFP-mut-Cterm, a nonbindingmutant version in which
the last three residues were mutated to alanine (NH2-GWFDAAA-COOH).
(B) Confocal images showing MDCK cells expressing either GFP-bcat-Cterm or GFP-mut-Cterm and endogenous subcellular localization of Scrib. GFP-bcat-
Cterm and endogenous Scrib were observed to colocalize in the cytosol (yellow); GFP-mut-Cterm, on the other hand, had no appreciable affect on the subcellular
localization of Scrib and did not colocalize with endogenous Scrib.
(C) Bar graph showing quantification of occludin length in a Ca2+-switch assay. Overexpressing GFP-bcat-Cterm caused a significant decrease in the average
length of occludin per cell, whereas overexpressing GFP-mut-Cterm had no effect (p < 0.05). The bar is the mean of at least three biological replicates, and error
bars represent SEM.(legend continued on next page)
Chemistry & Biology
b-Catenin Helps Maintain Tight Junctions
824 Chemistry & Biology 20, 816–827, June 20, 2013 ª2013 Elsevier Ltd All rights reserved

Chemistry & Biology
b-Catenin Helps Maintain Tight Junctions
Nevertheless, the PDZ proteins that interact with b-catenin
each exhibit distinct expression profiles across different cell
types and tissues (Table S3). It is therefore likely that the tissue-
specific expression of these PDZ proteins affects the role that
b-catenin plays in each cell type. For example, Magi-1, Magi-2,
Magi-3, and Scrib help recruit synaptic vesicles to the neuronal
synapse (Ito et al., 2012; Oliva et al., 2012; Roche et al., 2002)
and all four of these proteins interact with b-catenin through their
PDZ domains (Table 1). As they are all expressed at moderate to
high levels in brain tissue (Table S3), these interactionsmay guide
the role that b-catenin plays at the synaptic junction. Indeed,
Magi-1 and Scrib have previously been shown to colocalized at
synapses with b-catenin in cultured rat hippocampal neurons
and b-catenin coimmunoprecipitates with Magi-1 and Scrib in
neuronal cell lysates (Nishimura et al., 2002; Perego et al.,
2000; Sun et al., 2009). Additionally, overexpressing the C-termi-
nal region of b-catenin blocks synaptic targeting of Magi-1,
suggesting that the PDZ-domain-mediated interaction with
b-catenin is critical for its synaptic targeting. In a similar fashion,
Dvl-1, Dvl-3, and TIP-1 play important roles inWnt signaling (Gao
and Chen, 2010; Zhang et al., 2008) and each of these proteins
interacts with b-catenin through their PDZ domains (Table 1).
We would therefore expect that these proteins would be coex-
pressed with b-catenin in cells regulated by Wnt signaling. It is
interesting to note that Dvl-1 is highly expressed in intestinal tis-
sue, Dvl-3 in colon tissue, and TIP-1 in bone (Table S3), where
Wnt signaling plays prominent and well-documented roles (Hae-
gebarth and Clevers, 2009; Kramer et al., 2010).
Based on the overrepresentation of tight junction proteins in
our interaction data set (MUPP1, Pard3, Scrib, Magi-1, Magi-3,
ZO-3, and ZO-1), we focused on four specific PDZ domain-con-
taining proteins—Magi-1, Pard3, ZO-3, and Scrib—to determine
if their interactions with b-catenin play a functional role in tight
junction biology. Tight junctions provide contacts between
neighboring epithelial cells and act as the primary barriers to
the diffusion of solutes through the intercellular space (Tsukita
et al., 2001). They differ both in composition and purpose from
adherens junctions, which usually occur basal to tight junctions.
PDZ-domain-containing proteins have been shown to play
crucial roles in the proper functioning of tight junctions. For
example, Mupp1 serves as a multivalent scaffold to recruit
several essential tight junction proteins, including claudin and
junctional adhesion molecule (Hamazaki et al., 2002).
To delineate the role of b-catenin in tight junction biology, we
first determined which PDZ domains in each protein interact
directly with b-catenin (Figures 3B–3D). Then, using these iso-
lated domains as dominant negatives, we assessed the effect
of disrupting these interactions on tight junction function. This
strategy has previously been used to study protein function:
overexpressing the PDZ domain of ZO-1 causes the full-length
(D) TEM images ofMDCK cells overexpressingGFP-mut-Cterm showed the prese
arrow), in sites of cell-cell contact. In contrast, cells overexpressing GFP-bcat-C
(E) MDCK cells expressing GFP-bcat-Cterm also exhibited a decreased ability t
Fibrinogen, MDCK cells overexpressing GFP-bcat-Cterm exhibited significantly le
no change in their adhesion properties relative to untransfected cells. The bar is
(F and G) Overexpressing GFP-bcat-Cterm caused significant increases in migrat
control. Each bar is the mean of at least three biological replicates, error bars
transfected parental cells (p < 0.05).
Chemistry & Biology 20,
protein to mislocalize and results in an epithelial to mesenchymal
transition (Reichert et al., 2000; Ryeom et al., 2000). Using
confocal microscopy, we found that overexpressing cognate
PDZ domains from all four proteins increased the cytoplasmic
localization of their endogenous, full-length proteins and caused
aberrant localization of occludin. In addition, TEERwas reduced,
indicating a loss in tight junction integrity. These effects were not
observed when a PDZ domain that does not recognize b-catenin
was overexpressed. From these studies, we conclude that PDZ-
domain-mediated interactions with b-catenin determine the sub-
cellular localization of these four tight junction proteins, that
these interactions play a direct role in maintaining the integrity
of tight junctions, and that several different interactions are prob-
ably occurring in the same cell at the same time.
Interestingly, the localization of b-catenin was not affected in
these experiments, suggesting that its localization to the plasma
membrane is not determined by its interactions with these PDZ
proteins. As membrane recruitment of tight junction proteins is
a necessary step in the formation or maintenance of tight junc-
tions, it is likely that specific interactions with b-catenin are
required (Tsukita et al., 2001). Moreover, as b-catenin is associ-
ated with the cytoskeleton, it may act as a scaffold that links tight
junction-associated PDZ proteins to the cytoskeleton.
The functional importance of these interactions is further un-
derscored by our investigations of adhesion, proliferation, and
migration. Using the same dominant-negative strategy, we
found that overexpressing PDZ domains derived from Magi-1,
Pard3, Scrib, and ZO-3 resulted in decreased adhesion to a va-
riety of extracellular matrices and increased rates of proliferation
and migration.
Taken together, our data show that b-catenin plays a role in
defining the subcellular localization of Magi-1, Pard3, ZO-3, and
Scrib and that these interactions contribute to the integrity of tight
junctions, promote cellular adhesion, and inhibit cellular prolifer-
ationandmigration. Theseobservationsareparticularly intriguing
because many colon cancer cells exhibit aberrant accumulation
of b-catenin in the cytosol. In keeping with this, we found that
Magi-1, Pard3, ZO-3, and Scrib were predominantly localized
at theplasmamembrane in normalMDCKcells butwere localized
in the cytosol of SW480 colon cancer cells. This suggests that the
buildup of cytoplasmic b-catenin in colon cancer cells causes the
observed mislocalization of tight-junction-associated PDZ pro-
teins and that this, in turn, promotes decreased adhesion and
increased proliferation and migration. This model is consistent
with a previous study showing that increased tight junction
permeability and decreased epithelial barrier function precede
the development of colon tumors (Soler et al., 1999). We submit
that the mislocalization of tight junction proteins caused by the
misregulation of b-cateninmay play a functional role in the devel-
opment of colon cancer and warrants further investigation.
nce of typical tight junctions (yellow arrow), as well as adherens junctions (white
term showed weaker or no tight junctions.
o attach to extracellular matrices. When grown on Fibronectin, Collagen I, or
ss adhesion, whereas MDCK cells expressing GFP-mut-Cterm showed little or
the mean of at least two biological replicates, and error bars represent SEM.
ion and invasion (p < 0.05); no significant change was observed with the mutant
represent the SEM, and asterisks denote a significant difference from mock-
816–827, June 20, 2013 ª2013 Elsevier Ltd All rights reserved 825

Chemistry & Biology
b-Catenin Helps Maintain Tight Junctions
SIGNIFICANCE
b-catenin has previously been shown to interact with a vari-
ety of PDZ domain-containing proteins through its C termi-
nus, linking it to a diverse set of biological processes. Using
protein microarrays comprising virtually every mouse PDZ
domain, we identified and validated 26 PDZ domain-
mediated interactions with b-catenin, 17 of which had not
previously been described. Most notably, four tight-junc-
tion-associated PDZ proteins—Scrib, Magi-1, Pard3, and
ZO-3—interact with b-catenin in vitro and colocalize with it
at the plasmamembrane inMDCKepithelial cells. Disrupting
these interactions affects cellular adhesion, proliferation,
and migration, consistent with a model in which b-catenin
plays an integral role in maintaining cell-cell contacts,
participating not only in adherens junctions but also in tight
junctions. These studies show that biochemical interactions
identified in vitro can be used to uncover biology, suggesting
that constructing microarrays of protein interaction do-
mains provides a viableway to segment the problem of iden-
tifying biologically meaningful protein-protein interactions
on a proteome-wide scale.
EXPERIMENTAL PROCEDURES
Expression Constructs
Myc- and HA-tagged expression vectors were constructed using the Invitro-
gen Gateway system. Full-length cDNA for human b-catenin (ID 6151332),
LIN7b (ID 430017), Grasp55 (ID 4298251), Tiam2 (ID 9021272), ZO-3 (ID
40008232), PDZk7 (ID 5179973), Pard3 (ID 30344732), Mast1 (ID 5200610),
Dvl3 (ID 5200610), OMP25 (ID 3952366), and Scrib (ID 100015198) were ob-
tained from Open Biosystems. PCR products were prepared for each coding
region beginning with the sequence CACCand endingwith a stop codon (UAA)
using PFU Ultra polymerase (Stratagene) and the following thermocycling pa-
rameters: 95�C, 5 min; followed by 38 cycles of 95�C, 30 s; 54�C, 30 s; 72�C,1 min; followed by a final 10 min incubation at 72�C. The resulting products
were transferred into the vector pENTR/D-TOPO by topoisomerase-I-medi-
ated directional cloning (Invitrogen). Each clone was verified by DNA
sequencing. The coding region for each PDZ domain or full-length protein
was transferred into Gateway compatible mammalian expression vectors
by l-recombinase-mediated directional subcloning and confirmed by
sequencing. Expression vectors that append amino-terminal Myc and HA
tags were based on pCMV-Myc and pCMV-HA (Clontech Laboratories).
Cell Lines and Reagents
MDCK, HEK293, and SW480 cells were obtained from American Type Culture
Collection and maintained in Dulbecco’s modified Eagle’s medium supple-
mented with 10% (v/v) fetal bovine serum, 2 mM glutamine, 100 IU/mL
penicillin, and 100 mg/mL streptomycin. The expression constructs were intro-
duced into cells using Lipofectamine 2000 (Invitrogen) according to the man-
ufacturer’s instructions.
Primary antibodies were obtained from the following sources: rabbit anti-
b-catenin (Cell Signaling Technology; cat. #9562), mouse anti-b-catenin (Cell
Signaling Technology; cat #2677), mouse anti-Myc (Cell Signaling Technology;
cat #2276), rabbit anti-HA (Cell Signaling Technology; cat #3724), mouse anti-
b-actin (Sigma-Aldrich; cat. #A1978), rabbit anti-Magi-1 (Santa Cruz Bio-
technology; cat. #sc-25663), rabbit anti-Pard3 (Santa Cruz Biotechnology;
cat #sc-98509), rabbit anti-Scrib (Santa Cruz Biotechnology; cat #sc-28737),
and rabbit anti-ZO-3 (Invitrogen, cat #36-4000).
Pull-Down Assays and Western Blotting
Cell extracts for His6 pull-down assays or coimmunoprecipitation (co-IP) as-
says were prepared by rinsing cells with cold PBS and then introducing cell
lysis buffer (Cell Signaling Technology; cat #9803) supplemented with 1 mM
826 Chemistry & Biology 20, 816–827, June 20, 2013 ª2013 Elsevier
phenylmethylsulfonyl fluoride and a cocktail of protease inhibitors (0.5 ml per
well of a six-well plate). Samples were incubated at 4�Con a rotating shaker for
15min, and insoluble material was removed by centrifugation at 12,0003 g for
15min at 4�C. For pull-down experiments, the supernatant wasmixed with pu-
rified His6-tagged PDZ domains immobilized on Ni-NTA Agarose (QIAGEN)
and incubated on a rotating shaker for 2 hr at 4�C. For co-IP experiments,
the supernatant was mixed either with an anti-Myc antibody (Santa Cruz
Biotechnology; cat #SC40) or an anti-HA antibody (Santa Cruz Biotechnology;
cat #SC7392) for 2 hr at 4�C. Protein A/G PLUS-Agarose (Santa Cruz Biotech-
nology) was then added and the slurry incubated for 1 hr on a rotating shaker at
4�C. For both the pull-down and co-IP experiments, the agarose beads were
washed four times with lysis buffer and then boiled for 5 min in NuPAGE SDS
sample buffer (Invitrogen) prior to analysis by western blotting.
For quantitative immunoblots, primary antibodies were detected using IR-
Dye 680-labeled goat-anti-rabbit immunoglobulin G (IgG) or IRDye 800-
labeled goat-anti-mouse IgG (LI-COR Biosciences) at 1:10,000 dilutions.
Bands were visualized and quantified using an Odyssey Infrared Imaging Sys-
tem (LI-COR Biosciences).
TEER Measurement
TEER of the MDCK cell monolayers was monitored using a MillicellERS (Milli-
pore) connected to a pair of chopstick electrodes according to manufacturer’s
instructions. MDCK cells expressing Myc-tagged PDZ domains or empty vec-
tor were seeded in 24-well millicell plates (Millipore) at a density of 13 105 cells
per well. After 3 days, electrical potentials obtained from blank inserts were
subtracted from those obtained from inserts with confluent monolayers.
Monolayer resistance values were multiplied by the membrane area and aver-
aged to calculate TEER (Udcm2).
SUPPLEMENTAL INFORMATION
Supplemental Information includes Supplemental Experimental Procedures,
six figures, and four tables and can be found with this article online at http://
dx.doi.org/10.1016/j.chembiol.2013.04.021.
ACKNOWLEDGMENTS
We thank Daniel Goodenough and Marc Kirschner for helpful discussions and
suggestions and Peter Sorger for generous use of his facilities. We also thank
the staff of the Harvard Center for Biological Imaging and the Nikon Imaging
Center at Harvard Medical School for help and support. This study was sup-
ported by awards from the WM Keck Foundation and the Camille and Henry
Dreyfus Foundation and by grants from the National Institutes of Health (R01
GM072872, R33 CA128726, and P50 GM068762). T.S.G. is a Human Frontier
Science Program fellow, and E.S.K. is a recipient of a National Science Foun-
dation graduate fellowship. The funders had no role in study design, data
collection and analysis, decision to publish, or preparation of the manuscript.
T.S.G., E.S.K., and G.M. conceived and designed the research; T.S.G. and
G.M. analyzed the data and wrote the manuscript; and T.S.G., E.S.K., M.C.,
and B.H.C. performed the experiments and analyzed the data.
Received: January 13, 2013
Revised: March 26, 2013
Accepted: April 18, 2013
Published: June 20, 2013
REFERENCES
Abbosh, P.H., and Nephew, K.P. (2005). Multiple signaling pathways converge
on beta-catenin in thyroid cancer. Thyroid 15, 551–561.
Axelrod, J.D., Miller, J.R., Shulman, J.M., Moon, R.T., and Perrimon, N. (1998).
Differential recruitmentofDishevelledprovidessignalingspecificity in theplanar
cell polarity and Wingless signaling pathways. Genes Dev. 12, 2610–2622.
Bilder, D., and Perrimon, N. (2000). Localization of apical epithelial determi-
nants by the basolateral PDZ protein Scribble. Nature 403, 676–680.
Blankesteijn, W.M., van Gijn, M.E., Essers-Janssen, Y.P., Daemen, M.J., and
Smits, J.F. (2000). Beta-catenin, an inducer of uncontrolled cell proliferation
Ltd All rights reserved

Chemistry & Biology
b-Catenin Helps Maintain Tight Junctions
and migration in malignancies, is localized in the cytoplasm of vascular endo-
thelium during neovascularization after myocardial infarction. Am. J. Pathol.
157, 877–883.
Chen, X., andMacara, I.G. (2006). Par-3 mediates the inhibition of LIM kinase 2
to regulate cofilin phosphorylation and tight junction assembly. J. Cell Biol.
172, 671–678.
Dennis, G., Jr., Sherman, B.T., Hosack, D.A., Yang, J., Gao, W., Lane, H.C.,
and Lempicki, R.A. (2003). DAVID: Database for Annotation, Visualization,
and Integrated Discovery. Genome Biol. 4, 3.
Dobrosotskaya, I.Y., and James, G.L. (2000). MAGI-1 interacts with beta-cat-
enin and is associated with cell-cell adhesion structures. Biochem. Biophys.
Res. Commun. 270, 903–909.
Fanning, A.S., and Anderson, J.M. (1999). PDZ domains: fundamental building
blocks in the organization of protein complexes at the plasma membrane.
J. Clin. Invest. 103, 767–772.
Furuse, M., Hirase, T., Itoh, M., Nagafuchi, A., Yonemura, S., Tsukita, S., and
Tsukita, S. (1993). Occludin: a novel integral membrane protein localizing at
tight junctions. J. Cell Biol. 123, 1777–1788.
Gao, C., and Chen, Y.-G. (2010). Dishevelled: The hub of Wnt signaling. Cell.
Signal. 22, 717–727.
Gavert, N., and Ben-Ze’ev, A. (2007). beta-Catenin signaling in biological con-
trol and cancer. J. Cell. Biochem. 102, 820–828.
Gottardi, C.J., and Gumbiner, B.M. (2004). Distinct molecular forms of beta-
catenin are targeted to adhesive or transcriptional complexes. J. Cell Biol.
167, 339–349.
Haegebarth, A., and Clevers, H. (2009). Wnt signaling, lgr5, and stem cells in
the intestine and skin. Am. J. Pathol. 174, 715–721.
Hamazaki, Y., Itoh, M., Sasaki, H., Furuse, M., and Tsukita, S. (2002). Multi-
PDZ domain protein 1 (MUPP1) is concentrated at tight junctions through its
possible interaction with claudin-1 and junctional adhesion molecule. J. Biol.
Chem. 277, 455–461.
Harris, T.J., and Peifer, M. (2005). Decisions, decisions: beta-catenin chooses
between adhesion and transcription. Trends Cell Biol. 15, 234–237.
Herynk, M.H., Tsan, R., Radinsky, R., and Gallick, G.E. (2003). Activation of c-
Met in colorectal carcinoma cells leads to constitutive association of tyrosine-
phosphorylated beta-catenin. Clin. Exp. Metastasis 20, 291–300.
Ito, H., Morishita, R., Sudo, K., Nishimura, Y.V., Inaguma, Y., Iwamoto, I., and
Nagata, K.i. (2012). Biochemical and morphological characterization of MAGI-
1 in neuronal tissue. J. Neurosci. Res. 90, 1776–1781.
Itoh, M., Nagafuchi, A., Moroi, S., and Tsukita, S. (1997). Involvement of ZO-1
in cadherin-based cell adhesion through its direct binding to alpha catenin and
actin filaments. J. Cell Biol. 138, 181–192.
Kanamori, M., Sandy, P., Marzinotto, S., Benetti, R., Kai, C., Hayashizaki, Y.,
Schneider, C., and Suzuki, H. (2003). The PDZ protein tax-interacting pro-
tein-1 inhibits beta-catenin transcriptional activity and growth of colorectal
cancer cells. J. Biol. Chem. 278, 38758–38764.
Kim, E., and Sheng, M. (2004). PDZ domain proteins of synapses. Nat. Rev.
Neurosci. 5, 771–781.
Kramer, I.,Halleux,C.,Keller,H., Pegurri,M.,Gooi, J.H.,Weber,P.B.,Feng,J.Q.,
Bonewald, L.F., and Kneissel, M. (2010). Osteocyte Wnt/b-catenin signaling is
required for normal bone homeostasis. Mol. Cell. Biol. 30, 3071–3085.
Kumar Kundu, J., Choi, K.Y., and Surh, Y.J. (2006). beta-Catenin-mediated
signaling: A novel molecular target for chemoprevention with anti-inflamma-
tory substances. Biochim. Biophys. Acta 1765, 14–24.
Morin, P.J. (1999). beta-catenin signaling and cancer. Bioessays 21, 1021–
1030.
Nath, N., Kashfi, K., Chen, J., and Rigas, B. (2003). Nitric oxide-donating
aspirin inhibits beta-catenin/T cell factor (TCF) signaling in SW480 colon can-
cer cells by disrupting the nuclear beta-catenin-TCF association. Proc. Natl.
Acad. Sci. USA 100, 12584–12589.
Nishimura, W., Yao, I., Iida, J., Tanaka, N., and Hata, Y. (2002). Interaction of
synaptic scaffolding molecule and b -catenin. J. Neurosci. 22, 757–765.
Chemistry & Biology 20,
Nourry, C., Grant, S.G., and Borg, J.P. (2003). PDZ domain proteins: plug and
play! Sci. STKE 2003, RE7.
Oliva, C., Escobedo, P., Astorga, C., Molina, C., and Sierralta, J. (2012). Role of
the MAGUK protein family in synapse formation and function. Dev. Neurobiol.
72, 57–72.
Perego, C., Vanoni, C., Massari, S., Longhi, R., and Pietrini, G. (2000).
Mammalian LIN-7 PDZ proteins associate with beta-catenin at the cell-cell
junctions of epithelia and neurons. EMBO J. 19, 3978–3989.
Piedra, J., Miravet, S., Castano, J., Palmer, H.G., Heisterkamp, N., Garcıa de
Herreros, A., and Dunach, M. (2003). p120 Catenin-associated Fer and Fyn
tyrosine kinases regulate beta-catenin Tyr-142 phosphorylation and beta-cat-
enin-alpha-catenin Interaction. Mol. Cell. Biol. 23, 2287–2297.
Ress, A., and Moelling, K. (2008). The PDZ protein erbin modulates catenin-
dependent transcription. Eur. Surg. Res. 41, 284–289.
Reichert, M., Muller, T., and Hunziker, W. (2000). The PDZ domains of zonula
occludens-1 induce an epithelial to mesenchymal transition of Madin-Darby
canine kidney I cells. Evidence for a role of beta-catenin/Tcf/Lef signaling.
J. Biol. Chem. 275, 9492–9500.
Roche, J.P., Packard, M.C., Moeckel-Cole, S., and Budnik, V. (2002).
Regulation of synaptic plasticity and synaptic vesicle dynamics by the PDZ
protein Scribble. J. Neurosci. 22, 6471–6479.
Ryeom, S.W., Paul, D., and Goodenough, D.A. (2000). Truncation mutants of
the tight junction protein ZO-1 disrupt corneal epithelial cell morphology.
Mol. Biol. Cell 11, 1687–1696.
Schneeberger, E.E., and Lynch, R.D. (2004). The tight junction: a multifunc-
tional complex. Am. J. Physiol. Cell Physiol. 286, C1213–C1228.
Shin, K., Fogg, V.C., and Margolis, B. (2006). Tight junctions and cell polarity.
Annu. Rev. Cell Dev. Biol. 22, 207–235.
Smith, K.J., Johnson, K.A., Bryan, T.M., Hill, D.E., Markowitz, S., Willson, J.K.,
Paraskeva, C., Petersen, G.M., Hamilton, S.R., Vogelstein, B., et al. (1993). The
APC gene product in normal and tumor cells. Proc. Natl. Acad. Sci. USA 90,
2846–2850.
Soler, A.P., Miller, R.D., Laughlin, K.V., Carp, N.Z., Klurfeld, D.M., and Mullin,
J.M. (1999). Increased tight junctional permeability is associated with the
development of colon cancer. Carcinogenesis 20, 1425–1431.
Song, L., and Gelmann, E. (2005). Interaction of b-catenin and TIF2/GRIP1 in
transcriptional activation by the androgen receptor. J. Biol. Chem. 280,
37853–37867.
Song, D.H., Sussman, D.J., and Seldin, D.C. (2000). Endogenous protein ki-
nase CK2 participates in Wnt signaling in mammary epithelial cells. J. Biol.
Chem. 275, 23790–23797.
Stiffler, M.A., Grantcharova, V.P., Sevecka, M., and MacBeath, G. (2006).
Uncovering quantitative protein interaction networks for mouse PDZ domains
using protein microarrays. J. Am. Chem. Soc. 128, 5913–5922.
Stiffler, M.A., Chen, J.R., Grantcharova, V.P., Lei, Y., Fuchs, D., Allen, J.E.,
Zaslavskaia, L.A., and MacBeath, G. (2007). PDZ domain binding selectivity
is optimized across the mouse proteome. Science 317, 364–369.
Sun, Y., Aiga, M., Yoshida, E., Humbert, P.O., and Bamji, S.X. (2009). Scribble
interacts with b-catenin to localize synaptic vesicles to synapses. Mol. Biol.
Cell 20, 3390–3400.
Theisen, C., Wahl, J., III, Johnson, K., and Wheelock, M. (2007). NHERF links
the N-cadherin/catenin complex to the platelet-derived growth factor receptor
to modulate the actin cytoskeleton and regulate cell motility. Mol. Biol. Cell 18,
1220–1232.
Tsukita, S., Furuse, M., and Itoh, M. (2001). Multifunctional strands in tight
junctions. Nat. Rev. Mol. Cell Biol. 2, 285–293.
Wan, H., Winton, H.L., Soeller, C., Tovey, E.R., Gruenert, D.C., Thompson,
P.J., Stewart, G.A., Taylor, G.W., Garrod, D.R., Cannell, M.B., and
Robinson, C. (1999). Der p 1 facilitates transepithelial allergen delivery by
disruption of tight junctions. J. Clin. Invest. 104, 123–133.
Zhang, J., Yan, X., Shi, C., Yang, X., Guo, Y., Tian, C., Long, J., and Shen, Y.
(2008). Structural basis of b-catenin recognition by Tax-interacting protein-1.
J. Mol. Biol. 384, 255–263.
816–827, June 20, 2013 ª2013 Elsevier Ltd All rights reserved 827