E-cadherin and β-catenin expression in well-differentiated and moderately-differentiated oral...
Transcript of E-cadherin and β-catenin expression in well-differentiated and moderately-differentiated oral...

EacPLa
b
c
d
e
AA
A
TtofciwaecSw©
K
I
Om
Hdf
0
British Journal of Oral and Maxillofacial Surgery 51 (2013) 149–156
Available online at www.sciencedirect.com
-cadherin and �-catenin expression in well-differentiatednd moderately-differentiated oral squamous cellarcinoma: relations with clinical variablesablo Rosado a, Paloma Lequerica-Fernández b, Soledad Fernández c, Eva Allonca d,ucas Villallaín e, Juan C. de Vicente e,∗
Department of Oral and Maxillofacial Surgery, Hospital de Cabuenes, Gijón, Asturias, SpainDepartment of Biochemistry, Hospital San Agustín, Avilés, SpainDepartment of Pathology, Hospital Universitario Central de Asturias, Oviedo, SpainInstituto Universitario de Oncología del Principado de Asturias, SpainDepartment of Oral and Maxillofacial Surgery, Hospital Universitario Central de Asturias, Oviedo, Spain
ccepted 24 March 2012vailable online 21 April 2012
bstract
he aim of this study was to establish the expression and localisation of E-cadherin and �-catenin in oral squamous cell carcinomas (SCC) sohat we could correlate the findings with prognostically-relevant clinicopathological variables. E-cadherin and �-catenin expression in normalral mucosa and in oral squamous cell carcinomas were examined immunohistochemically, and their association with clinicopathologicalactors and prognosis were then analysed in 69 patients who had been operated on for oral SCC. E-cadherin expression was found in all 69ases: in 11 cases (16%) it was weak; in 21 (30%) moderate, and in 37 (54%) high. �-Catenin expression was found in 64 cases (93%):n 18 cases (26%) cell-membrane expression was weak; in 26 (38%) it was moderate; in 19 (28%) it was high, and in one case (1%) thereas cytoplasmic staining. No nuclear staining was detected. E-cadherin was significantly associated with histological grade (p = 0.002) and
lcohol consumption (p = 0.05), and �-catenin was significantly associated with nodal stage (p = 0.02), TNM stage (p = 0.009), and E-cadherinxpression (p = 0.01). However, none of them were independent prognostic factors in the disease-specific survival analysis. E-cadherin islosely linked to �-catenin expression in oral SCC and to tumour differentiation. Alcohol consumption could increase the aggressiveness of
CC, leading to reduced expression of E-cadherin. �-catenin could be an early marker for the identification of occult metastases in patientsith oral SCC.2012 The British Association of Oral and Maxillofacial Surgeons. Published by Elsevier Ltd. All rights reserved.
eywords: E-cadherin; �-Catenin; Oral squamous cell carcinoma; Immunohistochemistry
f
ntroductionral squamous cell carcinoma (SCC) is the sixth most com-on cancer in the world, and its prognosis depends on several
∗ Corresponding author at: Department of Oral and Maxillofacial Surgery,ospital Universitario Central de Asturias, Faculty of Medicine, c/ Cate-rático José Serrano s/n. 33006, Oviedo, Spain. Tel.: +34 85 103638;ax: +34 985103673.
E-mail address: [email protected] (J.C. de Vicente).
fnttmwsma
266-4356/$ – see front matter © 2012 The British Association of Oral and Maxillofaciahttp://dx.doi.org/10.1016/j.bjoms.2012.03.018
actors, including the size of the tumour, the degree of dif-erentiation, and the stage. The presence of cervical lymphode metastases is the most important prognostic indica-or, but they cannot always be predicted from the size ofhe primary cancer. It is interesting, therefore, to identify
olecular markers that would enable the selection of patientsith occult lymph node metastases. The process of metasta-
is is a cascade of linked sequential steps, and one of theost important steps is the weakening of cell adhesion. Cell
dhesion molecules, to which class cadherins and catenins
l Surgeons. Published by Elsevier Ltd. All rights reserved.

1 and Maxillofacial Surgery 51 (2013) 149–156
bcle
caiptcnt�
dtctcmusTsorm
twws
M
P
TwtaSbsrttAotopg3
Table 1Clinical features in 69 patients with oral squamous cell carcinoma.
Variable No. (%) of patients
Age (years):Median (range) 61 (24–87)
Sex:Male 54 (78)Female 15 (22)
Site:Lip 4 (6)Tongue 29 (42)Floor of mouth 18 (26)Gum 8 (12)Buccal 6 (9)Palate 4 (6)
T stage:T1 19 (28)T2 28 (41)T3 6 (9)T4 16 (23)
N stage:N0 44 (64)N1 13 (19)N2 12 (17)N3 0
TNM stage:I 14 (20)II 19 (28)III 12 (17)IV 24 (35)
Tumour differentiation:Poor 0
T
Ttpo6tarmcife
I
Tcs
50 P. Rosado et al. / British Journal of Oral
elong, are thought to have a key role both in cell–cell andell–extracellular matrix unions. E-cadherin, the main epithe-ial cell cadherin, plays a part in adherent junctions, whichstablish cell–cell contacts.
The intracellular domain of E-cadherin binds � and �-atenins, which in turn bind to �-catenin, and it attaches toctin filaments in the cell cytoskeleton. Whenever �-catenins dislocated from E-cadherin, it may accumulate in cyto-lasm and move to the nucleus of the cell, where it can activatehe transcription of genes involved in the development ofancer. When E-cadherin is normally expressed, therefore,ot only does it lead to the stabilisation of adherent junc-ions, but it also prevents the cytoplasmic accumulation of-catenin.
Reduced E-cadherin expression correlates with advancedisease in carcinomas of the breast, prostate and bladder, buthe correlation is weaker in the case of carcinomas of theolon and stomach, and squamous cell carcinomas (SCC) ofhe head and neck.1 Loss of E-cadherin expression has beenorrelated with poorly differentiated tumours,2 lymph nodeetastases, and poor survival.3 However, Ukpo et al.4 were
nable to find any association between E-cadherin expres-ion and the incidence of metastases in oropharyngeal SCC.he role of �-catenin in oral SCC is not yet well under-tood, and the clinical relevance of �-catenin expression inral SCC is controversial. Some studies have related theeduced membranous expression to poor survival and nodaletastases.The aim of the present study was to find out whether
he immunoexpression of E-cadherin and �-catenin inell-differentiated and moderately differentiated oral SCCere associated with clinicopathological variables and
urvival.
ethod
atients
he study was based on a retrospective cohort of 69 patientsith oral SCC who were randomly selected from those being
reated in the Department of Oral and Maxillofacial Surgeryt the Hospital Universitario Central de Asturias (Oviedo,pain). They had all been operated on with curative intentetween January 1990 and December 1992. Their details arehown in Table 1. The stage of disease was established afteresection of the tumour according to the TNM system ofhe International Union Against Cancer (6th ed). History ofobacco and alcohol use was taken from medical records.lcohol consumption was described in terms of beer, wine,r spirits. A drinker was defined as someone who statedhat they had consumed four or more drinks of any type
f alcoholic beverage/day for at least 10 years. For com-arability, one drink is equivalent to ethanol 14 g, whichenerally corresponds to beer 330 ml, wine 150 ml, and spirits6 ml.ottu
Moderate 14 (20)Good 55 (80)
issue microarray
he oral SCC tissue microarray was built with the approval ofhe institutional review board from archived formalin-fixed,araffin-embedded, tissue samples (Department of Pathol-gy of the Hospital Universitario Central de Asturias) from9 surgical patients. At least 3 samples were taken from donorissue blocks from each patient using a 2-mm punch to give
full picture of each case. In addition, the tissue microar-ay also contained morphologically normal samples of oralucosa, which were acquired from patients who did not have
ancer but required oral surgery. To check the histopatholog-cal diagnosis and the adequacy of tissue sampling, a sectionrom each microarray was stained with haematoxylin andosin and examined by light microscopy.
mmunohistochemistry
he oral SCC tissue microarray was examined immunohisto-hemically for E-cadherin and �-catenin expression. Briefly,ections of the microarray 4 �m thick were cut and driedn capillary-gap glass slides (Dako Cytomation). The sec-
ions were deparaffinised with standard xylene and hydratedhrough graded alcohols into water. Antigen was retrievedsing Target Retrieval Solution pH 6.0 in PTLink (Dako).
and M
SscImtSwtaaPf
tcdaaao
poisho(ade
S
ASssfepats
R
Ee
Ttpc
ce
Eo
EwiwEw
O(mel(
Ec
Tismrawda(
israE
C
T5ta(pa
P. Rosado et al. / British Journal of Oral
lides were stained at room temperature on an automatictaining workstation (Dako Autostainer Plus) with mono-lonal mouse antihuman E-cadherin (Upstate Biotechnologync., Lake Placid, NY) at 1/25 dilution for 20 min, and FLEXonoclonal mouse antihuman beta-catenin at 1/200 dilu-
ion for 30 min, using the Dako EnVision Flex + Visualisationystem (Dako Autostainer). They were finally counterstainedith haematoxylin for 1 min. The slides were then dehydrated
hrough graded alcohols and mounted with a coverslip using standard medium. Negative controls with omission of thentiserum from the primary incubation were also included.ositive controls (sample of normal oral epithelium obtainedrom healthy subjects) were used.
The slides were viewed randomly, without clinical data, bywo of the authors. They disagreed in 5 cases, which were dis-ussed until a consensus was achieved. In cases of persistentifferences between the authors, the sections were studied by
third independent observer and the majority decision wasccepted. Cytoplasmic-positive immunostaining was seen inll tumour cells with equal intensity within a given specimenf tumour.
The intensity of staining differed between individual sam-les of tumour, so immunostaining was scored based onlyn the percentage of stained cells, and subsequently dividednto three categories: low expression (score 1: <33% oftained cells), moderate expression (score 2: 34–66%) andigh expression (score 3: >67% of stained cells). In the casef �-catenin, cytoplasmic (score 4: cytoplasmic) and nuclearscore 5: nuclear) staining were also recorded. After a firstnalysis, and for statistical purposes, the staining data wereivided into reduced (score 1 + 2) or maintained (score 3)xpression.
tatistical analyses
ll statistical analyses were made with the help of thetatistical Package for the Social Sciences (SPSS ver-ion 18.0, Polar Engineering and Consulting). The chiquare test or Fisher’s exact test were used as appropriateor the comparison of categorical variables. Time-to-vent analyses were calculated using the Kaplan–Meierroduct-limit estimate. Differences between times werenalysed using the log-rank method. All tests were two-ailed. Probabilities of 0.05 or less were accepted asignificant.
esults
xpression of E-cadherin and β-catenin in normalpithelium from the oral cavity
he localisation of these proteins in normal epithelium fromhe oral cavity was analysed in samples obtained fromatients who did not have cancer. Both �-catenin and E-adherin expression were detected in basal and suprabasal
smr
axillofacial Surgery 51 (2013) 149–156 151
ell layers in a cell membrane pattern, whereas the mostxternal and differentiated layers were not stained.
-cadherin and β-catenin expression in specimens ofral SCC
-cadherin was expressed in all 69 oral SCC. This expressionas heterogeneous: in 11 (16%) it was weak; in 21 (30%)
t was moderate, and in 37 (54%) it was high. E-cadherinas strongly expressed in the cell membrane. In addition,-cadherin expression was negligible in keratinised nests ofell-differentiated tumours (Fig. 1).�-Catenin was expressed in 64 (93%) of the 69 cases.
f these, 18 (26%) had weak cell-membrane expression; 2638%) had moderate; and in 19 (28%) it was high. Cytoplas-ic staining was seen in 1 case (1%). �-Catenin was strongly
xpressed in the cell membrane but its expression was neg-igible in keratinised nests of well-differentiated tumoursFig. 2). No nuclear staining was detected.
-cadherin and β-catenin expression andlinicopathological variables (Tables 2 and 3)
here was no significant relation between E-cadherinmmunostaining and clinical variables such as age, sex,ite of tumour, recurrence, size of tumour (T), neck nodeetastases, TNM stage, or tobacco (p = 0.82). However,
educed immunostaining for E-cadherin was significantlyssociated with poor histological grade (p = 0.002). Ofell-differentiated tumours, 64% expressed E-cadherin, asid 14% of moderately differentiated tumours. There waslso a significant association with alcohol consumptionp = 0.05).
There was no significant relation between immunostain-ng with �-catenin and clinical variables such as age, sex,ize of tumour, recurrences, tobacco, or alcohol. However,educed expression of �-catenin was significantly associ-ted with nodal stage (p = 0.03) TNM stage (p = 0.009), and-cadherin expression (p = 0.02).
orrelation with the course of the disease
he mean and median follow-up of the whole group were5 and 53 months, respectively (range 10–128). Duringhis period, 28 patients (41%) developed local recurrence,nd 25 (36%) regional nodal metastases. Neither E-cadherinp = 0.68), nor �-catenin (p = 0.99) expression were inde-endent prognostic factors in the disease-specific survivalnalyses. Variables significantly associated with disease-
pecific survival were size of tumour (p < 0.0005), neck nodeetastases (p = 0.001), stage of disease (p = 0.01), and recur-ent disease (p < 0.0005).

152 P. Rosado et al. / British Journal of Oral and Maxillofacial Surgery 51 (2013) 149–156
Table 2Correlation between E-cadherin expression and clinicopathological variables.
Variable No. of patients E-cadherin-stained cells
<33% 34–66% >66% p value
Sex:Male 54 10 16 28 0.54Female 15 1 5 9
Site:Lip 4 2 1 1 0.87Tongue 29 5 9 15Floor of mouth 18 2 6 10Gum 8 1 2 5Buccal 6 1 2 3Palate 4 0 1 3
T stage:T1 19 2 5 12 0.93T2 28 6 8 14T3 6 1 2 3T4 16 2 6 8
N stage:N0 44 6 15 23 0.77N1 13 2 4 7N2 12 3 2 7
TNM stage:I 14 0 4 10 0.44II 19 5 6 8III 12 1 4 7IV 24 5 7 12
Tumour differentiation:Good 55 6 14 35 0.002Moderate 14 5 7 2
Recurrence:No 40 5 11 24 0.49Yes 29 6 10 13
Tobacco:No 22 3 6 13 0.82Yes 47 8 15 24
Alcohol:No 24 4 3 17 0.05
D
EiiicerS
thieStmt
tmchortHcooldewt
Yes 45 7
iscussion
-cadherin is thought to function as a tumour suppressor, ast suppresses invasion and metastases.5 The mechanisms fornhibition of E-cadherin include loss of heterozygosity andnactivating mutations of the gene, epigenetic silencing at theancer site, transcriptional repression, cadherin replacement,ndocytosis, and proteolytic processing. Several studies haveeported epigenetic silencing as a common feature in oralCC.6
In the present study we have analysed the expression andhe distribution of E-cadherin in primary oral SCC immuno-istochemically. Thirty-seven of 69 cases (54%) showedntense staining of tumour cells compared with those in whichxpression was reduced. These findings suggest that oralCC cells show heterogeneity in their potential to express
his molecule. The hypothetical reversibility of E-cadherinethylation could explain both the heterogeneous reduc-
ion of E-cadherin expression in metastatic cancer6 and
mti
18 20
he re-expression of E-cadherin in lymph node metastasesentioned in some studies.7 We found a significant asso-
iation between the immunoexpression of E-cadherin andistological grade. These findings are consistent with previ-us studies in which poorly differentiated tumours showededuced expression of this molecule,2,8 even emphasisinghe absence of fully undifferentiated tumours in our sample.owever, other authors9,10 found no relation between E-
adherin expression and histopathological differentiation inral and laryngeal cancer. This could mean that, irrespectivef the fact that all head and neck SCC are the same histopatho-ogical entity, they may behave differently depending on theiverse anatomical locations within the area, and even differ-nt tumours may behave differently. We found no associationith long-term survival or the presence of lymph node metas-
ases, in contrast to other studies.3,8 However, it should be
entioned that Kurtz et al.,1 examined a series of 45 SCC ofhe oral cavity, hypopharynx, and larynx with similar find-ngs to us; Ukpo et al.,4 studied 154 cases of oropharyngeal

P. Rosado et al. / British Journal of Oral and Maxillofacial Surgery 51 (2013) 149–156 153
Table 3Correlation between �-catenin expression and clinicopathological variables.
Variable No. of patients �-Catenin-stained cells
Reduced < 66% Maintained > 66% p value
Sex:Male 54 41 13 0.23Female 15 9 6
Site:Lip 4 3 1 0.86Tongue 29 23 6Floor of mouth 18 11 7Gum 7 5 2Buccal 6 4 2Palate 4 3 1
T stage:T1 19 11 8 0.27T2 28 20 8T3 6 5 1T4 16 14 2
N stage:N0 44 28 16 0.03N1 12 11 1N2 13 11 2
TNM stage:I 14 6 8 0.009II 19 12 7III 12 11 1IV 24 21 3
Tumour differentiation:Good 55 38 17 0.21Moderate 14 12 2
Recurrence:No 40 27 13 0.32Yes 29 23 6
Tobacco:No 22 14 8 0.28Yes 47 36 11
Alcohol:
StaiueosS
gsocsrhomi
�aIrrtoctetfiaca
No 24 16
Yes 45 34
CC, and Liu et al.9 examined 83 oral SCC, and none ofhem found any association between E-cadherin expressionnd the presence of lymph node metastases. The discrepancyn the results may lie in the fact that different antibodies weresed in these studies, but may also reflect biological differ-nces among different tumours. This suggests that there arether, potentially more important, mechanisms of metasta-is in well-differentiated and moderately differentiated oralCC.
Tobacco and alcohol have classically been involved in theenesis of oral SCC. Although epidemiological studies havehown a close relation between consumption of alcohol andral cancer, the biological basis of this relation has not beenlearly identified.11 To the best of our knowledge, the presenttudy provides the first description of the association betweeneduced expression of E-cadherin and alcohol intake. Weypothesise that alcohol may increase the aggressiveness
f cancer, which leads to reduced expression of adhesionolecules such as E-cadherin, and therefore increases thenvasive potential of the tumour.
pie
8 0.4611
The loss of E-cadherin increases the presence of soluble-catenin in the cytosol, but the Wnt/�-catenin pathway isctivated only when the degradation of �-catenin is altered.t is hardly surprising, therefore, that in the present study theeduced expression of E-cadherin is significantly related toeduced expression of �-catenin. This degradation processakes place at the degradation complex, which is composedf Axin and APC.12 When accumulated in the cytosol, �-atenin is transported into the cell nucleus where it binds tohe Tcf factor and activates certain genes that increase prolif-ration and malignancy. We could summarise the changes inhe Wnt/�-catenin pathway that are accounted for in cancer asollows: overexpression of Wnt proteins or repression of theirnhibitors, mutation of axin, mutations of the APC gene, andctivating mutations of the gene �-catenin/CTNNB1. In anyase, these processes will result in increased transcriptionalctivity of the �-catenin/Tcf product. The Wnt/�-catenin
athway is altered by mutation of APC, �-catenin or axinn variable percentages in many tumours. In oral SCC no rel-vant mutations of these genes have been found to date.13,14
154 P. Rosado et al. / British Journal of Oral and Maxillofacial Surgery 51 (2013) 149–156
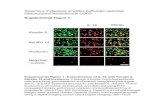
Fig. 1. Immunohistochemical study of E-cadherin in oral squamous cellcarcinoma. E-cadherin is expressed homogeneously in the cell membrane:(2
Haplt
dooi
Fcm
wrtanooof fully undifferentiated tumours in our sample could jus-
A) strong, (B) moderate, and (C) weak, expression (original magnification00×).
owever, loss of heterozygosity of the APC gene seems to be common event15,16 that has been associated with both poorrognosis and heavy smoking.17 More recently, Sogabe et al.inked the methylation of SFRP genes with the activation ofhe Wnt/�-catenin pathway in these patients.18
In the present paper we have studied the expression andistribution of �-catenin immunohistochemically in primary
ral SCC. �-catenin is expressed at the cell membrane, but itccasionally moves to the cytoplasm or the nucleus, resultingn staining at these levels. In our study �-catenin expressiontrt
ig. 2. Immunohistochemical study of �-catenin in oral squamous cellarcinoma. (A) Strong, (B) moderate, and (C) weak, expression (originalagnification 200×).
as reduced in 50 tumours (73%), figures similar to thoseeported by Bagutti et al.,19 and Gao et al.,20 but higher thanhose noted by Laxmidevi et al.,21 Cytoplasmic staining waslso seen in 1 case (1%), while there were no cases ofuclear staining. Unlike in other tumours, nuclear stainingf �-catenin is a rare phenomenon in oral SCC and, when itccurs, it is usually in undifferentiated tumours.3 The absence
ify this absence of nuclear expression. Other authors haveeported incidences of reduced catenin expression similar tohose found in our study.3,9,22

and M
besnTodvfaefiaEe
iseorca
efmw
A
TfI
R
1
1
1
1
1
1
1
1
1
1
2
2
P. Rosado et al. / British Journal of Oral
Several studies have shown significant correlationsetween metastases in the cervical lymph nodes and reducedxpression of �-catenin.9,23 In the present study we havehown that immunoexpression of �-catenin decreases sig-ificantly in patients with lymph node metastases and highNM stage. However, no relation was found to survivalr recurrence, as other authors have also reported.1,18 Toate, only Liu et al. have found a direct relation with sur-ival in a study with 82 patients9; Pukkila et al.24 alsoound that nuclear �-catenin expression predicted short over-ll survival. As far as the association between E-cadherinxpression and survival is concerned, some authors1,8,22 haveound a significant association between the degree of stain-ng and survival. Conversely, Ukpo et al.4 found no suchssociation, which was also present in our series. The use of-cadherin as a prognostic indicator, therefore, needs furthervaluation.
A limitation of this study is the sample size, which limitsts power. However, most of the studies discussed here hadample sizes similar to, or even smaller than, ours. We alsoxamined tissue microarray cores instead of whole sectionsf tumour. It is possible that a small tissue punch may noteflect the entire tumour, and so some variation in stainingould have been missed.4 However, we used 2 mm punches,nd we studied at least 3 samples from each patient.
In conclusion, E-cadherin is closely linked to �-cateninxpression in oral SCC. E-cadherin is related to tumour dif-erentiation and alcohol intake. �-Catenin could be an earlyarker for the identification of occult metastases in patientsith oral SCC.
cknowledgements
his work was supported by a grant for scientific researchrom the Ministry of Health, Spain (Instituto de Salud CarlosII, PI070675).
eferences
1. Kurtz KA, Hoffman HT, Zimmerman MB, Robinson RA. DecreasedE-cadherin but not beta-catenin expression is associated with vascularinvasion and decreased survival in head and neck squamous carcinomas.Otolaryngology-Head and Neck Surgery 2006;134:142–6.
2. Downer CS, Speight PM. E-cadherin expression in normal, hyperplasticand malignant oral epithelium. European Journal of Cancer Part B: OralOncology 1993;29B:303–5.
3. Tanaka N, Odajima T, Ogi K, Ikeda T, Satoh M. Expression of E-cadherin, alpha-catenin, and beta-catenin in the process of lymph nodemetastasis in oral squamous cell carcinoma. British Journal of Cancer2003;89:557–63.
4. Ukpo OC, Thorstad WL, Zhang Q, Lewis Jr JS. Lack of associationof cadherin expression and histopathologic type, metastasis, or patient
outcome in oropharyngeal squamous cell carcinoma: a tissue microarraystudy. Head and Neck Pathology 2011 [Epub ahead of print, Nov 10].5. Takeichi M. Cadherins in cancer: implications for invasion and metasta-sis. Current Opinion in Cell Biology 1993;5:806–11.
2
axillofacial Surgery 51 (2013) 149–156 155
6. Kudo Y, Kitajima S, Ogawa I, Hiraoka M, Sargolzaei S,Keikhaee MR, et al. Invasion and metastasis of oral cancercells require methylation of E-cadherin and/or degradation ofmembranous beta-catenin. Clinical Cancer Research 2004;10:5455–63.
7. Hung KF, Chang CS, Liu CJ, Lui MT, Cheng CY, Kao SY. Differen-tial expression of E-cadherin in metastatic lesions comparing to primaryoral squamous cell carcinoma. Journal of Oral Pathology and Medicine2006;35:589–94.
8. Mattijssen V, Peters H, Schalkwijk L, Manni JJ, vant’t Hof-GrootenboerB, de Mulder PH, et al. E-cadherin expression in head and neck quamouscell carcinomas is associated with clinical outcome. International Journalof Cancer 1993;55:580–5.
9. Liu LK, Jiang XY, Zhou XX, Wang DM, Song XL, Jiang HB.Upregulation of vimentin and aberrant expression of E-cadherin/beta-catenin complex in oral squamous cell carcinomas: correlation withthe clinicopathological features and patient outcome. Modern Pathology2010;23:213–24.
0. Rodrigo JP, Dominguez F, Suárez V, Canel M, Secades P, Chiara MD.Focal adhesion kinase and E-cadherin as markers for nodal metastasisin laryngeal cancer. Archives of Otolaryngology-Head and Neck Surgery2007;133:145–50.
1. Figuero-Ruiz E, Carretero-Peláez MA, Cerero-Lapiedra R, Esparza-Gómez G, Moreno-López LA. Effects of the consumption of alcoholin the oral cavity: relationship with oral cancer (in Spanish and English).Medicina Oral 2004;9:14–23.
2. Hart M, Concordet JP, Lassot I, Albert I, del los Santos R, DurandH, et al. The F-box protein beta-TrCP associates with phosphory-lated beta-catenin and regulates its activity in the cell. Current Biology1999;9:207–10.
3. Iwai S, Katagiri W, Kong C, Amekawa S, Nakazawa M, Yura Y. Mutationsof the APC, beta-catenin, and axin 1 genes and cytoplasmic accumula-tion of beta-catenin in oral squamous cell carcinoma. Journal of CancerResearch and Clinical Oncology 2005;131:773–82.
4. Kok SH, Lee JJ, Hsu HC, Chiang CP, Kuo YS, Kuo MY. Mutations of theadenomatous polyposis coli gene in areca quid and tobacco-associatedoral squamous cell carcinomas in Taiwan. Journal of Oral Pathology andMedicine 2002;31:395–401.
5. Kannan S, Yokozaki H, Jayasree K, Sebastian P, Mathews A, AbrahamEK, et al. Infrequent loss of heterozygosity of the major tumour sup-pressor genes in Indian oral cancers. International Journal of Oral andMaxillofacial Surgery 2002;31:414–8.
6. Tandle A, Sanghavi V, Saranath D. Infrequent loss of heterozygosity atadenomatous polyposis coli gene locus in Indian oral cancers. CancerLetters 2000;157:155–60.
7. Mao EJ, Schwartz SM, Daling JR, Beckmann AM. Loss of heterozygosityat 5q21-22 (adenomatous polyposis coli gene region) in oral squamouscell carcinoma is common and correlated with advanced disease. Journalof Oral Pathology and Medicine 1998;27:297–302.
8. Sogabe Y, Suzuki H, Toyota M, Ogi K, Imai T, Nojima M,et al. Epigenetic inactivation of SFRP genes in oral squa-mous cell carcinoma. International Journal of Oncology 2008;32:1253–61.
9. Bagutti C, Speight PM, Watt FM. Comparison of integrin, cadherin, andcatenin expression in squamous cell carcinomas of the oral cavity. Journalof Pathology 1998;186:8–16.
0. Gao S, Eiberg H, Krogdahl A, Liu CJ, Sørensen JA. Cyto-plasmic expression of E-cadherin and beta-catenin correlated withLOH and hypermethylation of the APC gene in oral squamouscell carcinomas. Journal of Oral Pathology and Medicine 2005;34:116–9.
1. Laxmidevi LB, Angadi PV, Pillai RK, Chandreshekar C. Aberrant �-catenin expression in the histologic differentiation of oral squamous cell
carcinoma and verrucous carcinoma: an immunohistochemical study.Journal of Oral Science 2010;52:633–40.2. Chow V, Yuen AP, Lam KY, Tsao GS, Ho WK, Wei WI. A comparativestudy of the clinicopathological significance of E-cadherin and catenins

1 and M
2
56 P. Rosado et al. / British Journal of Oral
(alpha, beta, gamma) expression in the surgical management of oraltongue carcinoma. Journal of Cancer Research and Clinical Oncology
2001;127:59–63.3. Bánkfalvi A, Krassort M, Végh A, Felszeghy E, Piffkó J. Derangedexpression of the E-cadherin/beta-catenin complex and the epidermalgrowth factor receptor in the clinical evolution and progression of oral
2
axillofacial Surgery 51 (2013) 149–156
squamous cell carcinomas. Journal of Oral Pathology and Medicine2002;31:450–7.
4. Pukkila MJ, Virtaniemi JA, Kumpulainen EJ, Pirinen RT, JohanssonRT, Valtonen HJ, et al. Nuclear beta catenin expression is related tounfavourable outcome in oropharyngeal and hypopharyngeal squamouscell carcinoma. Journal of Clinical Pathology 2001;54:42–7.










![27. PASSAGE OF PARTICLES THROUGHMATTERpdg.lbl.gov/2007/reviews/passagerpp.pdf · 27. PASSAGE OF PARTICLES THROUGHMATTER ... 82] Moderately ... distributions and dielectric-response](https://static.fdocument.org/doc/165x107/5aee194c7f8b9ae531913db8/27-passage-of-particles-passage-of-particles-throughmatter-82-moderately.jpg)




![27. PASSAGE OF PARTICLES THROUGHMATTER27.2. Electronic energy loss by heavy particles [1{8] Moderately relativistic charged particles other than electrons lose energy in matter primarily](https://static.fdocument.org/doc/165x107/6040be6be1d8b644047832e7/27-passage-of-particles-throughmatter-272-electronic-energy-loss-by-heavy-particles.jpg)

