Breaking Down the Barrier to Rapid Anti - bacterial Drug ...
Differences in amyloid-β clearance across mouse and human blood–brain barrier models: Kinetic...
Transcript of Differences in amyloid-β clearance across mouse and human blood–brain barrier models: Kinetic...

Q3
lable at ScienceDirect
Neuropharmacology xxx (2014) 1e11
123456789101112131415161718192021222324252627282930313233343536373839404142434445464748495051525354
55
NP5381_proof ■ 25 January 2014 ■ 1/11
Contents lists avai
Neuropharmacology
journal homepage: www.elsevier .com/locate/neuropharm
56575859606162636465666768697071727374757677
Differences in amyloid-b clearance across mouse and humanbloodebrain barrier models: Kinetic analysis and mechanisticmodeling
Hisham Qosa a, Bilal S. Abuasal a, Ignacio A. Romero b, Babette Weksler c,Pierre-Oliver Couraud d, Jeffrey N. Keller e, Amal Kaddoumi a,*aDepartment of Basic Pharmaceutical Science, College of Pharmacy, University of Louisiana at Monroe, Monroe, LA, USAb The Open University, Life Sciences, Milton Keynes, UKcWeill Medical College, Medicine/Heme-Onc, New York, NY, USAd INSERM U1016, Institut Cochin, Paris, Francee Pennington Biomedical Research Center, Louisiana State University, Baton Rouge, LA, USA
787980
8182838485868788899091a r t i c l e i n f o
Article history:Received 6 September 2013Received in revised form9 December 2013Accepted 13 January 2014
Keywords:Amyloid-bBloodebrain barrierClearanceMechanistic model
Abbreviations: AD, Alzheimer’s disease; Ab, amylosor protein; BBB, bloodebrain barrier; BCI, brain cleaindex; CSF, cerebrospinal fluid; IDE, insulin degradinlipoprotein receptor-related protein-1; P-gp, P-glycopcation end product; TCA, trichloroacetic acid.* Corresponding author. Department of Basic Pharm
Pharmacy, University of Louisiana at Monroe, 1800 BieUSA. Tel.: þ1 318 342 1460; fax: þ1 318 342 1737.
E-mail address: [email protected] (A. Kaddoum
0028-3908/$ e see front matter � 2014 Published byhttp://dx.doi.org/10.1016/j.neuropharm.2014.01.023
9293949596979899
100101
Please cite this article in press as: Qosa, H.,Kinetic analysis and mechanistic modeling,
a b s t r a c t
Alzheimer’s disease (AD) has a characteristic hallmark of amyloid-b (Ab) accumulation in the brain. Thisaccumulation of Ab has been related to its faulty cerebral clearance. Indeed, preclinical studies that usedmice to investigate Ab clearance showed that efflux across bloodebrain barrier (BBB) and brain degra-dation mediate efficient Ab clearance. However, the contribution of each process to Ab clearance remainsunclear. Moreover, it is still uncertain how species differences between mouse and human could affect Abclearance. Here, a modified form of the brain efflux index method was used to estimate the contributionof BBB and brain degradation to Ab clearance from the brain of wild type mice. We estimated that 62% ofintracerebrally injected 125I-Ab40 is cleared across BBB while 38% is cleared by brain degradation.Furthermore, in vitro and in silico studies were performed to compare Ab clearance between mouse andhuman BBB models. Kinetic studies for Ab40 disposition in bEnd3 and hCMEC/D3 cells, representativein vitro mouse and human BBB models, respectively, demonstrated 30-fold higher rate of 125I-Ab40uptake and 15-fold higher rate of degradation by bEnd3 compared to hCMEC/D3 cells. Expression studiesshowed both cells to express different levels of P-glycoprotein and RAGE, while LRP1 levels were com-parable. Finally, we established a mechanistic model, which could successfully predict cellular levels of125I-Ab40 and the rate of each process. Established mechanistic model suggested significantly higher ratesof Ab uptake and degradation in bEnd3 cells as rationale for the observed differences in 125I-Ab40disposition between mouse and human BBB models. In conclusion, current study demonstrates theimportant role of BBB in the clearance of Ab from the brain. Moreover, it provides insight into the dif-ferences between mouse and human BBB with regards to Ab clearance and offer, for the first time, amathematical model that describes Ab clearance across BBB.
� 2014 Published by Elsevier Ltd.
102
103104id-b; APP, amyloid-b precur-rance index; BEI, brain effluxg enzyme; LRP1, low densityrotein; RAGE, advanced gly-
aceutical Science, College ofnville Dr., Monroe, LA 71201,
i).
Elsevier Ltd.
105106107108109110111112113114115116
et al., Differences in amyloidNeuropharmacology (2014),
1. Introduction
Amyloid-b peptides (Ab) are by-products of neuronal meta-bolism that have been linked to the pathogenesis of Alzheimerdisease (AD) (Selkoe, 1993). Cerebral levels of these peptides areregulated by their production rate from proteolytic degradation ofamyloid precursor protein (APP), influx from plasma that is medi-ated mainly by receptor for advanced glycation end product (RAGE)(Deane et al., 2003), and by their clearance from the brain (Sommer,2002). In AD, the rate of cerebral accumulation of Ab peptides,mainly Ab40 and Ab42, is accelerated resulting in toxic aggregates ofdifferent sizes ranging from soluble oligomers to insoluble plaques
117118119
-b clearance across mouse and human bloodebrain barrier models:http://dx.doi.org/10.1016/j.neuropharm.2014.01.023

H. Qosa et al. / Neuropharmacology xxx (2014) 1e112
1234567891011121314151617181920212223242526272829303132333435363738394041424344454647484950515253545556575859606162636465
66676869707172737475767778798081828384858687888990919293949596979899
100101102103104105106107108109110111112113114115116117118119120121122123124125126127128129130
NP5381_proof ■ 25 January 2014 ■ 2/11
(Jan et al., 2010). In very rare cases of AD (familial AD), Ab accu-mulation is related to its overproduction (Citron et al., 1992).However, mounting evidence suggests that Ab accumulation in thebrain of late-onset “sporadic” AD patients and in some cases offamilial AD is related to its impaired clearance from brain (Deaneand Zlokovic, 2007). Moreover, a previous study has shown thatlate-onset AD is associated with 30% decrease in the clearance of Abwhile the production rate did not differ between control and ADindividuals (Mawuenyega et al., 2010). Clearance of Ab from thebrain takes place by three pathways, transport across the bloodebrain barrier (BBB) (Deane et al., 2009), degradation in the braintissue (Iwata et al., 2000), and bulk flow of cerebrospinal fluid (CSF)(Silverberg et al., 2003). It is estimated that the clearance rate ofAb40 across BBB is 6-fold higher than its clearance rate through bulkflow of CSF (Bell et al., 2007); however, the relative contribution ofbrain degradation was not determined.
Clearance of Ab40 across the BBB has been extensively studiedover the past decade where many contributing transporters/re-ceptors at the BBB have been identified (Deane et al., 2009). Inaddition, accelerated cerebral accumulation of Ab40 due toimpaired clearance across the BBB has also been demonstrated tosignificantly affect its deposition and plaque formation in the brainof AD patients (Bell and Zlokovic, 2009). The main infrastructure ofthe BBB that regulates Ab40 clearance is the endothelial cells liningthe brain capillaries. Endothelial cells are connected to each otherby strong tight junctions and they are anchored to a continuousbasement membrane that is supported by perivascular end-feet ofthe astrocytes forming a physical barrier for the movement ofcompounds (Ballabh et al., 2004). Given the important contributionof endothelial cells to the function of the BBB, transport of Ab40across these cells is a crucial step in the clearance of Ab40. As apeptide, Ab40 has poor passive membrane permeability and it de-pends on transport system to pass across the endothelial cells ofBBB (Banks et al., 2003). Ab40 is known to be a substrate for manyreceptors and transporters at the BBB such as low density lipo-protein receptor-related protein-1 (LRP1) and P-glycoprotein (P-gp)that contribute to its clearance from the brain to blood (Cirrito et al.,2005; Shibata et al., 2000), and RAGE that is involved in Ab40 influxfrom blood to brain (Deane et al., 2003).
Mouse is a widely used animal model to study cerebral clear-ance of Ab40. Available studies, independently (Barten et al., 2005;Cirrito et al., 2003; Mawuenyega et al., 2010), demonstrated asignificant difference in Ab40 clearance rate between mice andhumans. In cognitively normal individuals, about 7% of Ab40 iscleared from the brain per hour (corresponding half-life of w10 h)and this percent decreased to 5.2% in patients with AD (corre-sponding half-life of w13 h) (Mawuenyega et al., 2010). On theother hand, the half-life of human Ab40 in mouse brain rangesfrom 0.63 h in Tg2576 mice to 2 h in PDAPP mice (Barten et al.,2005; Cirrito et al., 2003). Thus, the rate of Ab40 clearance frommouse brain is at least 5-fold higher than human brain. However,differences in the rate of Ab40 clearance by degradation and/ortransport across the BBB between mouse and human remainunknown.
Evaluation of kinetic parameters of Ab40 uptake, efflux anddegradation by endothelial cells is essential to understand keysteps involved in Ab40 clearance across the BBB. To date, availablestudies have modeled Ab40 clearance in vivo across the mouse BBBto calculate Ab40 kinetic parameters (Bell et al., 2007; Kandimallaet al., 2005; Shibata et al., 2000). However, the calculated pa-rameters represented overall clearance across BBB and do notdistinguish between clearance via transport or degradation. Toestimate the kinetic parameters of the several processes involvedin Ab40 clearance including uptake, efflux and degradation acrossthe BBB, in vitro models of BBB are preferred. Moreover, as Ab40
Please cite this article in press as: Qosa, H., et al., Differences in amyloidKinetic analysis and mechanistic modeling, Neuropharmacology (2014),
disposition in brain endothelial cells involves several processesthat simultaneously take place, it is difficult to use a conventionalMichaeliseMenten approach to estimate Ab40 disposition kineticparameters by these cells. Therefore, utilization of mechanisticmodels should provide a better approach to estimate and under-stand Ab40 kinetic disposition in endothelial cells (Poirier et al.,2008).
Accordingly and given the important role of the BBB in theclearance of Ab40, we hypothesized that BBB plays the major role inbrain Ab40 clearance compared to Ab40 brain degradation, and thatthe higher clearance rate of Ab40 from mouse brain compared tohuman brain is due to differences in Ab40 clearance across the BBB.Three aims were set to examine this hypothesis, 1) investigate thein vivo contribution of the BBB to Ab40 clearance relative to its braindegradation, 2) compare mechanisms and rate of Ab40 clearancebetween mouse and human BBB in vitro models, and 3) establish amechanistic kinetic model that describe differences in Ab40disposition between mouse and human endothelial cells.
2. Material and methods
2.1. Animals
C57BL/6 male mice (6e7 weeks old; Harlan Laboratories, Houston, TX) werekept under standard environmental conditions (22 �C, 35% relative humidity, 12 hdark/light cycle) with free access to tap water and standard rodent food. All animalexperiments were approved by the Institutional Animal Care and Use Committee ofthe University of Louisiana at Monroe. All surgical procedures were consistent withthe IACUC policies and procedures.
2.2. Brain efflux index (BEI) study
In vivo Ab40 clearance was investigated using the BEI method as describedpreviously (Qosa et al., 2012). In brief, a stainless steel guide cannula was implantedstereotaxically into the right caudate nucleus of mice that had been anesthetizedwith intraperitoneal xylazine and ketamine (20 and 125mg/kg, respectively) (HenrySchein Inc., NY). After 12 h recovery period, animals were re-anesthetized and tracerfluid (0.5 ml) containing 125I-Ab40 (30 nM, PerkinElmer, MA) and 14C-inulin (0.02 mCi,American Radiolabeled Chemicals, MO) prepared in extracellular fluid buffer (ECF)was administered. Thirty minutes post 125I-Ab40 injection (Cirrito et al., 2005;Shibata et al., 2000); brain tissues were rapidly collected for 125I-Ab40 analysis. Tocharacterize role of P-gp and LRP1, 0.5 ml of ECF containing valspodar (40 mM;XenoTech, KS), a well-established P-gp inhibitor, or RAP (1 mM; Oxford BiomedicalResearch, MI), an LRP1 inhibitor, were intracerebrally administered 5 min prior to125I-Ab40 injection.
2.3. Calculation of in vivo 125I-Ab40 clearance
Calculations of 125I-Ab40 clearance were performed as described previously(Qosa et al., 2012; Shibata et al., 2000). Using the trichloroacetic acid (TCA) pre-cipitation assay intact (precipitate) and degraded (supernatant) 125I-Ab40 weredetermined in brain tissue using a Wallac 1470 Wizard Gamma Counter (Perki-nElmer Inc., Waltham, MA). 14C-inulin in precipitate and supernatant were alsodetermined using a Wallac 1414 WinSpectral Counter (PerkinElmer Inc.). To calcu-late the total clearance of 125I-Ab40 from the brain, a modified equation of the BEImethod (Qosa et al., 2012) was used to calculate brain clearance index (BCITotal(%)),i.e. clearance across BBB and brain degradation (Equation (1)). Clearance of 125I-Ab40across BBB (BCIBBB(%)) and brain degradation (BCIDegradation(%)) were defined byEquations (2) and (3), respectively.
BCITotalð%Þ ¼ 100�24
�Amount of intact 125I�Ab40 in the brain
Amount of 14C�inulin in the brain
��Amount of intact 125I�Ab40 injected into the brain
Amount of 14C�inulin injected
�� 100
35 (1)
BCIBBBð%Þ ¼ 100�24�Amount of total125I�Ab40 in the brainðintact and degradedÞ
Amount of 14C�inulin in the brain
��Amount of intact 125 I�Ab40 injected into the brain
Amount of 14C�inulin injected
� � 100
35 (2)
BCIDegradationð%Þ ¼ BCITotalð%Þ � BCIBBBð%Þ (3)
2.4. Cell culture
The mouse (bEnd3) and human (hCMEC/D3) brain endothelial cells wereused as representative models for mouse and human BBB, respectively. bEnd3,passage 25e35, were cultured in DMEM supplemented with 10% fetal bovineserum (FBS), penicillin G (100 units/ml) and streptomycin (100 mg/ml). hCMEC/
-b clearance across mouse and human bloodebrain barrier models:http://dx.doi.org/10.1016/j.neuropharm.2014.01.023

H. Qosa et al. / Neuropharmacology xxx (2014) 1e11 3
1234567891011121314151617181920212223242526272829303132333435363738394041424344454647484950515253545556575859606162636465
66676869707172737475767778798081828384858687888990919293949596979899
100101102103104105106107108109110111112113114115116117118119120121122123124125126127128129130
NP5381_proof ■ 25 January 2014 ■ 3/11
D3, passage 25e35, were cultured in EBM-2 medium supplemented with 1 ng/mlhuman basic fibroblast growth factor (Sigma-Aldrich, MO), 10 mM HEPES, 1%chemically defined lipid concentrate (Gibco, NY), 5 mg/ml ascorbic acid, 1.4 mMhydrocortisone, 1% penicillin-streptomycin and 5% of heat-inactivated FBS“gold”. Cultures were maintained in a humidified atmosphere (5%CO2/95% air) at37 �C.
2.5. Uptake and degradation of 125I-Ab40 by bEnd3 and hCMEC/D3 cells
To study uptake and degradation of 125I-Ab40, cells were seeded at a density of5 � 104 per well in 24-well plate, and maintained for 3e5 days. Uptake anddegradation of 125I-Ab40 were evaluated for 15 min and 12 h in bEnd3 and hCMEC/D3 cells, respectively. These time points were determined from 125I-Ab40 depletionstudies. In addition, initial studies to confirm the indivisible 125I-label fromAb40, andthe integrity of 125I-Ab40 over different time points were performed by incubation of0.1 nM of 125I-Ab40 in media without cells, and by 16% bis-tris gel electrophoresis.The results demonstrated insignificant dissociation of 125I from Ab40 during theinvestigated time points (Supplementary Fig. 1a), and the absence of dimers orhigher oligomer formation at 12 h, the duration of the uptake study (SupplementaryFig. 1b). Uptake studies were initiated by addition of fresh warmmedium containing0.1 nM of 125I-Ab40. For inhibition studies, RAP (1 mM), RAGE-IgG (5 mg/ml, SantaCruz Biotechnology Inc., CA, USA) and valspodar (5 mM) were pre- and co-incubatedwith 125I-Ab40 at 37 �C. At the end of incubation period, media were collected andcells were washed twice with ice-cold PBS containing 0.2% BSA to minimize non-specific binding of 125I-Ab40, and once with warm PBS. Cells were dissolved in200 ml RIPA buffer containing complete mammalian protease inhibitors for 20 minon ice with shaking. Intact and degraded 125I-Ab40 were measured in media and celllysates as described below. Total amount of protein in each sample was determinedusing BCA protein assays. Net uptake of 125I-Ab40 by the cells was normalized to thetotal amount of cellular protein.
2.6. 125I-Ab40 analysis
For all in vitro experiments, intact and degraded 125I-Ab40 were measured in themedium and cells. Total 125I-Ab40 was determined by counting sample radioactivity.Degraded 125I-Ab40 was measured in the supernatant following precipitation withTCA (20%) in 1:1 ratio (v/v) (Qosa et al., 2012). The intact fraction was calculated bysubtracting degraded 125I-Ab40 from total 125I-Ab40. ELISA kits (SensoLyte Anti-Human beta-Amyloid 1e40, Anaspec, CA) were used to calculate exact startingconcentrations for all in vitro experiments, and to confirm 125I-Ab40 degradationobtained from TCA assay. For ELISA analysis, 100 ml of each sample were used tomeasure intact 125I-Ab40. All cpm values of 125I-Ab40 were converted to fmole basedon the specific activity of 125I-Ab40, g-counter efficiency and fmole versus cpmcalibration curve obtained by ELISA assay (data not show).
2.7. Localization of Ab40-HiLyte Fluor into acidic organelles
For imaging studies, cells were plated on 8-well chambered glass slides (LAB-Tek, Nalge Nunc, NY) at 12,000 cells/cm2, and allowed to grow overnight. bEnd3and hCMEC/D cells were incubated with Ab40-HiLyte Fluor (1 nM, Alexa-Fluor,Anaspec, CA) for 15 min and 12 h, respectively. To track Ab40-HiLyte Fluor inacidic organelles, LysoTracker Red (Invitrogen, OR, USA), a weakly basic fluo-rophore that selectively accumulates in acidic organelles of the endosomal/lyso-somal pathway, was added at 75 nM to the incubation media 45 min prior to theend of Ab40-HiLyte Fluor incubation in hCMEC/D cells, and 30 min prior to theaddition of Ab40-HiLyte Fluor and during incubation in bEnd3 cells. At the end ofthe incubation periods, cells were washed and fixed with 4% formaldehyde for5 min on ice. Cells were mounted with DAPI. Images for Ab40-HiLyte Fluor andLysoTracker Red were captured using a Zeiss LSM 5 Pa confocal microscopeequipped with 488 nm Argon laser and 543 nm HeNe Laser using a 63� oil im-mersion objective lens with numerical aperture ¼ 1.4 (Carl Zeiss MicroImagingLLC, Thornwood, NY).
2.8. 125I-Ab40 transport across bEnd3 and hCMEC/D3 cells
For transport studies, transwell polyester membrane inserts, 6.5 mm diam-eter with 0.4 mm pores (Corning, NY), were coated with collagen (150 mg/ml).Cells were plated onto coated inserts at a seeding density of 50,000 cells/cm2,medium was changed every other day. Trans-epithelial electrical resistance(TEER) was measured using an EVOM epithelial volt-ohmmeter with STX2 elec-trodes (World Precision Instruments, FL). According to the value of TEER(w35 U cm2) both cell lines were used for 125I-Ab40 transport experiments on day6. Apical to basolateral (A / B) transport studies were initiated by addition of0.1 nM 125I-Ab40 and 0.05 mM 14C-inulin to the apical compartment, whilebasolateral to apical (B / A) transport studies were initiated by addition of0.1 nM 125I-Ab40 and 0.05 mM 14C-inulin to the basolateral compartment. At theend of incubation period (30 min for bEnd3 and 12 h for hCMEC/D3 cells), mediain both compartments and cells were separately collected for 125I-Ab40 analysisand inulin measurement. The clearance quotients of A / B (125I-Ab40 CQA/B) andB / A (125I-Ab40 CQB/A) transports were calculated using the following equa-tions (Nazer et al., 2008):
Please cite this article in press as: Qosa, H., et al., Differences in amyloidKinetic analysis and mechanistic modeling, Neuropharmacology (2014),
125I� Ab40 CQA/B ¼125I�Ab40 in basolateral compartment
125I�Ab40 total�14C�inulin in basolateral compartment
� (4)
� �
14C�inulin total
125I� Ab40 CQB/A ¼�
125I�Ab40 in apical compartment125 I�Ab40 total
��
14C�inulin in apical compartment14C�inulin total
� (5)
where 125I-Ab40 total is the total intact cpm in the apical and basolateral compart-ments, as well as cpm remaining in cells. 14C-inulin total is the total inulin dpm in theapical and basolateral compartments. To evaluate the effect of different inhibitors on125I-Ab40 transport and degradation across cell monolayer valspodar (5 mM), RAP(1 mM), and RAGE-IgG (5 mg/ml) were pre- and co-incubated with 125I-Ab40.
2.9. Western blotting
For western blot analysis of P-gp, LRP1 and RAGE in bEnd3 and hCMEC/D3 cells,25 mg of cellular protein was resolved on 7.5% SDS-polyacrylamide gels and trans-ferred onto nitrocellulose membrane. Membranes were blocked with 2% BSA andincubated overnight with monoclonal antibodies for LRP1 (Calbiochem, NJ), P-gp (C-219, Covance Research Products, MA), RAGE (N-16) or b-actin (C-11) (Santa Cruz) atdilutions 1:200, 1:1500, 1:200 and 1:3000, respectively. For protein detection, themembranes were washed and incubated with HRP-labeled secondary IgG antibodyfor LRP1 and P-gp (anti-mouse) and RAGE and b-actin (anti-goat) (Santa Cruz) at1:5000 dilutions. The bands were visualized using a SuperSignal West Femtodetection kit (Thermo Scientific, IL). Quantitative analysis of the immunoreactivebands was performed using a GeneSnap luminescent image analyzer (ScientificResources Southwest, TX) and band intensities were measured by densitometricanalysis.
2.10. Modeling of 125I-Ab40 disposition in bEnd3 and hCMEC/D3 cells
Intracellular concentration of intact 125I-Ab40 is controlled by three active pro-cesses; uptake, efflux and degradation. To establish a mechanistic model that de-scribes these processes, cells were grown in 24-well plates and maintained asdescribed above. Experiments were initiated by addition of fresh warm mediacontaining 125I-Ab40. bEnd3 cells were incubated at 37 �C for 5, 15, 30, 60 and120 min with different 125I-Ab40 concentrations of 0.4, 0.8, 1.5, 3, 8 nM, whilehCMEC/D3 cells were incubated at 37 �C for 0.5, 1, 6, 12 and 24 h with 125I-Ab40concentrations of 0.8, 1.5, 3, 6, 12 nM. At the end of each incubation period, mediawere removed and cells were washed and lysed. Concentrations of intact anddegraded 125I-Ab40 were measured in both medium and cells. To calculate effluxparameters, bEnd3 and hCMEC/D3 cells were preloaded with different concentra-tions of 125I-Ab40, as above, for 15 min and 12 h, respectively. At the end of the in-cubation period, culture media were removed, cells were washed and fresh warmmediawere added to the cells. Cells were incubated for 1, 3, and 5 min at 37 �C. Afterthat, media were collected and cells were washed and lysed. 125I-Ab40 in media andcells were analyzed and efflux rates (fmole/min) of intact 125I-Ab40 were plottedagainst intracellular 125I-Ab40 concentrations (nM). Efflux parameters were used asfixed values in building the model to simplify modeling procedure by decreasing thenumber of parameters to be estimated. For intracellular volume estimation(w0.18 ml), the protein content of endothelial cells was converted to volume using apreviously published constant of 3 ml/mg protein (Brillault et al., 2008). At allexamined concentrations, time versus intact 125I-Ab40 concentration in intracellularcompartment was modeled according to Equation (6):
dCcelldt
¼ Vmax;uptake � Cmed
Km;uptake þ Cmed� Vmax;efflux � CcellKmax;efflux þ Ccell
� CL (6)
and the time versus intact 125I-Ab40 concentration in medium compartment wasmodeled according to Equation (7):
dCmeddt
¼ �Vmax;uptake � Cmed
Km;uptake þ Cmedþ Vmax;efflux � CcellKmax;efflux þ Ccell
(7)
whereas, change in the concentration of degraded 125I-Ab40 in cells overtime wasdefined by Equation (8):
dDcelldt
¼ CL � Ccell (8)
In all equations, Vmax was expressed in fmole/min, Km in nM, and CL in nl/min.The mechanistic in vitro model was implemented in MatLab version 7.10 (Math-Works, MA, USA) to simultaneously estimate Vmax, Km and CL of 125I-Ab40 uptake,while Vmax and Km of the efflux were fed to the model as fixed values that wereestimated from the efflux studies. All data points were fitted in a single step usingEquation (6) with triplicates for each of the five time points for each concentration.SDs were calculated for all parameters obtained from individual experiments andcoefficients of variation (CVs) for each parameter were subsequently calculated andexpressed as a percentage to assess the quality of the parameter (Poirier et al., 2008).
-b clearance across mouse and human bloodebrain barrier models:http://dx.doi.org/10.1016/j.neuropharm.2014.01.023

H. Qosa et al. / Neuropharmacology xxx (2014) 1e114
1234567891011121314151617181920212223242526272829303132333435363738394041424344454647484950515253545556575859606162636465
66676869707172737475767778798081828384858687888990919293949596979899
100101102103104105106107108109110111112113114115116117118119120121122123
NP5381_proof ■ 25 January 2014 ■ 4/11
Please cite this article in press as: Qosa, H., et al., Differences in amyloidKinetic analysis and mechanistic modeling, Neuropharmacology (2014),
2.11. Statistical analysis
Unless otherwise indicated, the data were expressed as mean � SEM. Theexperimental results were statistically analyzed for significant difference using two-tailed Student’s t-test for 2 two groups, and one-way analysis of variance (ANOVA)for more than two groups analysis. Values of P < 0.05 were considered statisticallysignificant.
3. Results
3.1. In vivo clearance of 125I-Ab40 across BBB
The first objective of current study was to estimate the specificcontribution of BBB to the clearance of 125I-Ab40 from the brains ofC57BL/6 mice. BCI method, a modified method for BEI, was used tocalculate total clearance, i.e. efflux across BBB and clearance bydegradation of 125I-Ab40 in mouse brain. BCITotal(%) demonstrated58.7 � 3.9% of administered 125I-Ab40 was cleared from mousebrain 30 min following 125I-Ab40 microinjection (Fig. 1A). BCIBBB(%)showed that 35.6 � 3.3% of injected 125I-Ab40, i.e. 62% of theBCITotal(%), was cleared across the BBB (Fig. 1B). On the other hand,23.1 � 2.3% of injected 125I-Ab40, i.e. 38% of BCITotal(%), was clearedby brain degradation (Fig. 1C). To elucidate the specific contributionof P-gp and LRP1 to BBB clearance and brain degradation of 125I-Ab40, BCITotal(%), BCIBBB(%) and BCIDegradation(%) of 125I-Ab40 weredetermined in the presence of specific P-gp and LRP1 inhibitors. P-gp and LRP1 inhibition caused 25% (43.9 � 4.8%, P < 0.05) and 49%(30.1 �1.7, P < 0.01) reduction, respectively, in 125I-Ab40 BCITotal(%)(Fig. 1A). Moreover, our results showed that although P-gp andLRP1 inhibition significantly decreased 125I-Ab40 BCIBBB(%) to18.3 � 1.5% (P < 0.01) and 22.9 � 1.7 (P < 0.05), respectively, onlyinhibition of LRP1 decreased 125I-Ab40 BCIDegradation(%) to7.2 � 0.4% (P < 0.001; Fig. 1B and C). Collectively, these resultsconfirm the significant contribution of BBB to cerebral clearance of125I-Ab40 compared to brain degradation, and signify the impor-tance of P-gp and LRP1 at the BBB in this process.
3.2. In vitro uptake of 125I-Ab40 by bEnd3 and hCMEC/D3 cells
Results from preliminary depletion studies showed that while2 h incubation time was sufficient for complete depletion of 125I-Ab40 from the medium of bEnd3 cells, more than 24 h incubationtimewas requiredwith hCMEC/D3 cells (Fig. 2). Consistent with thedepletion study, bEnd3 cells showed very rapid uptake anddegradation of 125I-Ab40 compared to hCMEC/D3 cells. As shown inFig. 3A, bEnd3 cellular level of intact 125I-Ab40 was 0.27� 0.02 fmol/mg after 15 min incubation, while 12 h was needed for hCMEC/D3to reach a similar cellular level of intact 125I-Ab40 (0.26 � 0.01 fmol/mg). The degradation of 125I-Ab40 by bEnd3 and hCMEC/D3,measured in the medium, reached 73.7 � 2.0 and 65.7 � 1.8% after15 min and 12 h of 125I-Ab40 addition, respectively (Fig. 3B). Inhi-bition of LRP1 by RAP significantly decreased the cellular level ofintact 125I-Ab40 in bEnd3 and hCMEC/D3 cells by 67% and 42%,respectively (P < 0.001, Fig. 3A). The degradation of 125I-Ab40 inbEnd3 and hCMEC/D3 was also decreased by 24% and 28%,respectively, by RAP (P< 0.01, Fig. 3B). On the other hand, inhibitionof RAGE significantly decreased the cellular level of intact 125I-Ab40by 75% in both cells (P< 0.001, Fig. 3A), while 125I-Ab40 degradationwas not altered by RAGE inhibition in both cells (P > 0.05, Fig. 3B).
Fig. 1. In vivo clearance of 125I-Ab40 from brain of C57BL/6 mice. Total brain clearance(BCITotal(%)) of 125I-Ab40 in control (CNTRL), with P-gp inhibitor valspodar (VALSP) orLRP1 inhibitor RAP (A), BBB clearance (BCIBBB(%)) of 125I-Ab40 in control and inhibitorgroups (B), clearance of 125I-Ab40 by brain degradation (BCIDegradation(%)) in control andinhibitor groups (C). Data represent mean � SEM for n ¼ 4; *P < 0.05, **P < 0.01 and***P < 0.001. NS is not significant.
124125126127128129130
-b clearance across mouse and human bloodebrain barrier models:http://dx.doi.org/10.1016/j.neuropharm.2014.01.023

Fig. 2. Depletion studies of 125I-Ab40 in bEnd3 and hCMEC/D3 cells over 24 h. Mediacontaining 1.5 nM 125I-Ab40 was added to cells in 24-well plate. Media were collectedafter 5, 15, 30 min and 1, 6, 12, 24 h to measure intact 125I-Ab40 remaining in media byELISA and radioactivity analyses. Figure inside inset demonstrates the linear range ofdepletion (0e0.5 h) observed with bEnd3.
Fig. 3. In vitro uptake and degradation of 125I-Ab40 by bEnd3 and hCMEC/D3 cells. A)In vitro uptake of intact 125I-Ab40 by bEnd3 and hCMEC/D3 cells. Cellular level of intact125I-Ab40 is expressed in fmole/mg protein following cells treatment with 0.1 nM 125I-Ab40 without (control; CNTRL) and with RAGE (RAGE-IgG), or P-gp (valspodar; VALSP),or LRP1 (RAP) inhibitors for 15 min and 12 h in bEnd3 and hCMEC/D3, respectively. B)Percent of degraded 125I-Ab40 in the media of bEnd3 and hCMEC/D3 cells followingtreatment with 0.1 nM of 125I-Ab40, with or without inhibitor. Data representmean � SEM from three independent experiments; *P < 0.05, **P < 0.01 and***P < 0.001.
H. Qosa et al. / Neuropharmacology xxx (2014) 1e11 5
1234567891011121314151617181920212223242526272829303132333435363738394041424344454647484950515253545556575859606162636465
66676869707172737475767778798081828384858687888990919293949596979899
100101102103104105106107108109110111112113114115116117118119120121122123124125126127128129130
NP5381_proof ■ 25 January 2014 ■ 5/11
Inhibition of P-gp by valspodar did not cause any significant changein 125I-Ab40 accumulation in bEnd3 cells (P> 0.05, Fig. 3A), whereasthis effect was obvious and significant in hCMEC/D3 where P-gpinhibition caused 15% increase in cellular levels of intact 125I-Ab40(P< 0.05, Fig. 3A). As expected, P-gp inhibition has no effect on 125I-Ab40 degradation in both cells (P> 0.05, Fig. 3B). In order to explainthe differences in 125I-Ab40 disposition between the two cell lines,expression studies were performed by Western blotting. Both celllines showed comparable levels of LRP1, significantly higher RAGEexpression in bEnd3 compared to hCMEC/D3, and higher expres-sion of P-gp in hCMEC/D3 compared to bEnd3 cells (Fig. 4).
3.3. Accumulation of Ab40 in acidic organelles
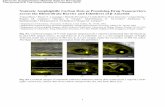
While the localization of Ab40 in endosomal/lysosomal organellshas been previously reported in bovine endothelial cells(Kandimalla et al., 2009), in this study, we have shown for the firsttime the endosomal/lysosomal localization of Ab40 in human andmouse brain endothelial cells, a pathway that is associated withdegradation preceded by peptide uptake. The cellular localizationof Ab40 was studied by monitoring cellular accumulation of Ab40-HiLyte Fluor in the acidic organelles. Ab40-HiLyte Fluor showed aclear co-localization with LysoTracker Red in bEnd3 and hCMEC/D3cells (Fig. 5). Pearson’s correlation coefficients of Ab40-HiLyte Fluorco-localization with LysoTracker Red were about 0.64 in both celllines, suggesting that uptaken Ab40 was directed to degradation viaendosomal/lysosomal pathway.
Additional experiments were performed to evaluate thecontribution of specific 125I-Ab40 degrading proteases to its totaldegradation relative to the lysosomal degradation. In these studies,the expression and activity of major Ab40 degrading enzymesincluding insulin-degrading enzyme (IDE) and neprilysin in bEnd3and hCMEC/D3 cells were evaluated. Expression studies wereachieved by Western blotting, and activity studies were examinedusing inhibition studies for each enzyme. The results demonstratedthat IDE and neprilysin are expressed in both cell lines withsignificantly higher expression in bEnd3 (Supplementary Fig. 2a). Inaddition, while the inhibition of IDE by bacitracin (1 mg/ml)significantly decreased Ab40 degradation in both cells, its contri-bution to the total degradation was low (20% and 12% in bEnd3 andhCMEC/D3, respectively (Supplementary Fig. 2b)). On the otherhand, the inhibition of neprilysin by thiorphan (2 mM) did not
Please cite this article in press as: Qosa, H., et al., Differences in amyloidKinetic analysis and mechanistic modeling, Neuropharmacology (2014),
show any significant effect on Ab40 degradation in both cells(Supplementary Fig. 2b). Neprilysin has low affinity for its physio-logical substrates (in the low mM range) (Shibata et al., 2000) andAb40 concentrations used in our experiments were in the low nMrange, thus it is more likely for Ab40 to bind its high affinity re-ceptors that mediate its endocytosis and non-specific lysosomaldegradation. Collectively, these findings demonstrate that both
-b clearance across mouse and human bloodebrain barrier models:http://dx.doi.org/10.1016/j.neuropharm.2014.01.023

Fig. 4. Expression of LRP1, RAGE and P-gp in bEnd3 and hCMEC/D3 cells. A) Westernblot analysis of LRP1, RAGE and P-gp protein expression in bEnd3 and hCMEC/D3 cells.B) Densitometry analyses showed similar expression level of LRP1 in two cells, higherRAGE expression in bEnd3 cells and higher P-gp expression in hCMEC/D3 cells. Datarepresent mean � SEM from three independent experiments; **P < 0.01, NS is notsignificant.
H. Qosa et al. / Neuropharmacology xxx (2014) 1e116
1234567891011121314151617181920212223242526272829303132333435363738394041424344454647484950515253545556575859606162636465
66676869707172737475767778798081828384858687888990919293949596979899
100101102103104105106107108109110111112113114115116117118119120121122123124125126127128129130
NP5381_proof ■ 25 January 2014 ■ 6/11
non-specific lysosomal degradation and specific protease depen-dent degradation are involved in Ab40 degradation by brain endo-thelial cells, however, with superior contribution of the non-specific lysosomal pathway.
3.4. 125I-Ab40 transport across endothelial cells
Results of transport studies showed A/ B transport of 125I-Ab40was about 2-fold higher across bEnd3 than hCMEC/D3 cells. CQA/B
of intact 125I-Ab40 was 0.62 � 0.06 and 0.32 � 0.02 across bEnd3and hCMEC/D3 cells, respectively (P < 0.01, Fig. 6A). RAGE inhibi-tion decreased A / B transport of 125I-Ab40 by 50% in bEnd3 and33% in hCMEC/D3 cells (P < 0.01, Fig. 6A). Consistent with the re-sults of the uptake study, inhibition of P-gp increased transport of125I-Ab40 from A / B by 22% in hCMEC/D3 while no alteration in125I-Ab40 transport in bEnd3 was observed (Fig. 6A). On the otherhand, 125I-Ab40 transport from B / A was more than 2-fold higherin hCMEC/D3 compared to bEnd3 (P < 0.01, Fig. 6B). This obser-vation can be explained by the fact that at 30min the concentrationof intact 125I-Ab40 available in bEnd3 for transport is lower than
Please cite this article in press as: Qosa, H., et al., Differences in amyloidKinetic analysis and mechanistic modeling, Neuropharmacology (2014),
that at 12 h in hCMEC/D3, which is consistent with the rapiddegradation of 125I-Ab40 seen in the depletion studies (Fig. 2). Inagreement with the role of LRP1, RAP decreased intact 125I-Ab40transport from B / A direction by 42% in both cells (P < 0.01,Fig. 6B). In addition, consistent with rapid depletion in bEnd3,degradation of 125I-Ab40 was significantly higher in bEnd3 thanhCMEC/D3 in both directions (P < 0.01, Fig. 6C and D). Inhibition ofRAGE, P-gp, or LRP1 did not affect 125I-Ab40 degradation in both celllines and in either direction (Fig. 6C and D).
3.5. Mechanistic model for Ab40 cellular disposition
Initial uptake studies of 125I-Ab40 (0.1 nM) over 2 and 24 h in-cubation in bEnd3 and hCMEC/D3, respectively, showed a saturableuptake process of 125I-Ab40 by both cells, with significantly higherrate of uptake in bEnd3 cells (Supplementary Fig. 3). On the otherhand, efflux studies demonstrated a saturable efflux process in bothcells with similar rates (Supplementary Fig. 4). Degradation studies,however, demonstrated unsaturable intracellular degradation of125I-Ab40 in both cells as the percentage of degraded 125I-Ab40 wereconstant (73.7 � 2.0 and 65.7 � 1.8% in bEnd3 and hCMEC/D3,respectively) as a function of concentration. High intracellularconcentration of Ab40 and presence of multiple processes that worksimultaneously necessitates the use of a mechanistic modelapproach to estimate kinetic parameters for each of these integratedprocesses. Kinetic parameter determination using a mechanisticmodel approach depends on measurement of 125I-Ab40 uptake anddegradation at multiple time points and for different 125I-Ab40concentrations at each time point. Based on our results of 125I-Ab40uptake and degradation in bEnd3 and hCMEC/D3 cells at differenttime points and 125I-Ab40 concentrations, we established a mecha-nistic two-compartment model that describes uptake, efflux anddegradation of 125I-Ab40 by endothelial cells. The model structureconsisted of saturable uptake and efflux processes in addition tolinear intracellular degradation process (Fig. 7A). Appropriateequations were fit to the mean 125I-Ab40 accumulation in endothe-lial cells versus time data plotted in Fig. 7 (BeE). Based on visualinspection of the residuals, coefficients of variation (CV%) of therecoveredparameters andAkaike’s information criterion values, themodel structure presented in Fig. 7A provided the best descriptionof the entire data set. This model scheme incorporated saturableuptake and efflux steps (Km,uptake, Vm,uptake andKm,efflux, Vm,efflux) anda linear intracellular degradation (CL). Estimates of kinetic param-eters obtained from the model are shown in Table 1. The modelrecovered good estimates of all parameters. Consistent with theobserved data, established model indicated a very rapid 125I-Ab40uptake in bEnd3 when compared to hCMEC/D3 cells with intrinsicuptake (Vmax/Km) frommedia of 35-fold higher in bEnd3. Moreover,this model showed 15-fold higher rate of intracellular degradation(CL) by bEnd3 cells. Estimated Kmvalues indicated that 125I-Ab40 hashigher affinity for uptake receptors in bEnd3 than in hCMEC/D3while it has similar affinity for efflux transporters/receptors(Table 1). Similarly, uptakewasmore efficient in bEnd3 thanhCMEC/D3 evident by the higher Vmax for 125I-Ab40 uptake in bEnd3 cellswhile the rate of efflux were comparable between the two cells.
4. Discussion
Efficient brain clearance of Ab40 is important to prevent its brainaccumulation (Mawuenyega et al., 2010). Therefore, quantitativeassessment of the contribution of different clearance pathways isimportant to elucidate key factors that could significantly enhancebrain Ab40 accumulation. AlthoughAb40 is less pathogenic thanAb42due to its low propensity to aggregate (Jan et al., 2010; Sommer,2002), Ab40 is found at several fold higher than Ab42 in the brain,
-b clearance across mouse and human bloodebrain barrier models:http://dx.doi.org/10.1016/j.neuropharm.2014.01.023

Fig. 5. Degradation of Ab40 within endosomal/lysosomal pathway of bEnd3 and hCMEC/D3 cells. Co-localization of intracellular Ab40-HiLyte Fluor and LysoTracker Red in endo-somal/lysosomal pathway of brain endothelial cells (bEnd3 and hCMEC/D3) analyzed using confocal immunoFluorescence microscope. bEnd3 cells and hCMEC/D3 were incubatedwith 1 nM of Ab40-HiLyte Fluor for 15 min or 12 h; respectively. DAPI stains nuclei. Lysosomes were labeled with LysoTracker Red for 45 min before the end of study. The yellow colorin the merged image represent co-localization of green (Ab40-HiLyte Fluor) and red (LysoTracker Red) fluorescence. (For interpretation of the references to colour in this figurelegend, the reader is referred to the web version of this article.)
Fig. 6. Transport of Ab40 across bEnd3 and hCMEC/D3 cell monolayers. Apical to basolateral (A / B) and basolateral to apical (B / A) permeability and degradation of 0.1 nM 125I-Ab40 across bEnd3 and hCMEC/D3 cells determined at 30 min and 12 h, respectively. Clearance quotient CQA/B and degradation of 125I-Ab40 across bEnd3 and hCMEC/D3 cells in theabsence (CNTRL) or presence of RAGE (RAGE-IgG) or P-gp (VALSP) inhibitors (A, C). CQB/A and degradation of 125I-Ab40 across bEnd3 and hCMEC/D3 in the absence (CNTRL) orpresence of LRP1 inhibitor (RAP) (B, D). Data represent mean � SEM from three independent experiments, *P < 0.05, **P < 0.01 and ***P < 0.001.
H. Qosa et al. / Neuropharmacology xxx (2014) 1e11 7
1234567891011121314151617181920212223242526272829303132333435363738394041424344454647484950515253545556575859606162636465
66676869707172737475767778798081828384858687888990919293949596979899
100101102103104105106107108109110111112113114115116117118119120121122123124125126127128129130
NP5381_proof ■ 25 January 2014 ■ 7/11
Please cite this article in press as: Qosa, H., et al., Differences in amyloid-b clearance across mouse and human bloodebrain barrier models:Kinetic analysis and mechanistic modeling, Neuropharmacology (2014), http://dx.doi.org/10.1016/j.neuropharm.2014.01.023

Fig. 7. Mechanistic modeling of Ab40 disposition by bEnd3 and hCMEC/D3 cells. Schematic description of proposed mechanistic model that describes 125I-Ab40 uptake, efflux anddegradation by endothelial cells (A). This mechanistic model contains two-compartments corresponding to the incubation media (Cmed, concentration of intact 125I-Ab40 in media innM), and intracellular space of the cells (Ccell, cellular concentration of intact 125I-Ab40 in nM; Dcell, cellular concentration of degraded 125I-Ab40 in nM). This model provides theoptimal model structure for 125I-Ab40 disposition in endothelial cells. Kinetic profiles of cellular levels of intact (B) and degraded (D) 125I-Ab40 in bEnd3 cells obtained whenmeasured in plated cells at 0.4 nM (C), 0.8 nM (:), 1.5 nM (;), 3 nM (A) and 6 nM (-) over 2 h incubation period; and cellular levels of intact (C) and degraded (E) 125I-Ab40 inhCMEC/D3 cells when measured in plated cells at 0.8 nM (C), 1.5 nM (:), 3 nM (;), 6 nM (A) and 12 nM (-) over 24 h incubation period. Lines represent the predicted cellularlevel obtained from the mechanistic two-compartment model. Observed data points are mean of triplicate measurements.
Table 1Kinetic apparent parameters of 125I-Ab40 disposition by hCMEC/D3 and bEnd3 cellsestimated from mechanistic model.
Parameter hCMEC/D3 bEnd3
Estimate CV% Estimate CV%
Km, uptake (nM) 49.1 0.1 19.8 14.3Vmax, uptake (fmole/min) 4.0 0.01 56.4 3.9Intrinsic uptake (nl/min) 81 2848Km, efflux (nM) 7.5 17.4 7.6 22.7Vmax, efflux (fmole/min) 0.12 12.2 0.14 17.1Intrinsic efflux (nl/min) 16 18CL (nl/min) 17 4.5 260 5
H. Qosa et al. / Neuropharmacology xxx (2014) 1e118
1234567891011121314151617181920212223242526272829303132333435363738394041424344454647484950515253545556575859606162636465
66676869707172737475767778798081828384858687888990919293949596979899
100101102103104105106107108109110111112113114115116117118119120121122123124125126127128129130
NP5381_proof ■ 25 January 2014 ■ 8/11
Please cite this article in press as: Qosa, H., et al., Differences in amyloidKinetic analysis and mechanistic modeling, Neuropharmacology (2014),
and its accumulation disrupts the integrity of BBB (Gregory andHalliday, 2005). Therefore, it is important to investigate clearanceof Ab40 across the BBB. Previous studies reported significantcontribution of the BBB to Ab40 clearance with less contribution ofCSF bulk flow; however, the role of brain degradation was notelucidated (Bell et al., 2007). In the current study, we estimatedcontribution of BBB and brain degradation to the total brain clear-ance of Ab40 in mouse brain using a modified form of the BEImethod. The BEI method depends on measuring intact 125I-Ab40remained in the brain after specific time of intracerebrally injected125I-Ab40, which does not only consider clearance across the BBB butalso brain degradation of 125I-Ab40. The modified method of BEIallowed quantification of BBB relative contribution (62%) and braindegradation (38%) to the total clearance of brain Ab40, excludingclearance via CSFflowwhichwas previouslyestimated to contributeby 10e15% of overall brain Ab40 clearance (Shibata et al., 2000). The
-b clearance across mouse and human bloodebrain barrier models:http://dx.doi.org/10.1016/j.neuropharm.2014.01.023

H. Qosa et al. / Neuropharmacology xxx (2014) 1e11 9
1234567891011121314151617181920212223242526272829303132333435363738394041424344454647484950515253545556575859606162636465
66676869707172737475767778798081828384858687888990919293949596979899
100
NP5381_proof ■ 25 January 2014 ■ 9/11
relative contribution of these Ab40 clearance pathways from thebrain is summarized in Fig. 8. Consistent with its function as Ab40efflux transporter (Cirrito et al., 2005), inhibition of P-gp decreasedthe clearance of Ab40 across the BBB without altering its braindegradation, while LRP1 inhibition by RAP decreased both, clear-ance across the BBB and brain degradation of Ab40. These results arein agreement with brain distribution and function of LRP1. LRP1 isexpressed in brain microvessels where it mediates intact Ab40clearance via receptor-mediated transcytosis (Castellano et al.,2012; Shibata et al., 2000), and in neurons and brain vascularsmoothmuscle cellswhere itmediates endocytosis and degradationof Ab40 (Fuentealba et al., 2010; Kanekiyo et al., 2012). Low-densitylipoprotein receptor (LDLR) family members have also been re-ported to mediate Ab40 degradation in particular cell types withinthe brain (Basak et al., 2012), and similar to LRP1, these LDLRmembers are subjected to RAP inhibition (Bu,1998). Thus, reductionin brain Ab40 degradation observed following RAP intracerebraladministration could be related to LRP1 inhibition and possiblyother LDLRs expressed in different brain cellular components.However, at the BBB, it is possible that RAP primarily inhibitedLRP1transport of Ab40 as proposed by a previous study where theinhibition of LRP1 at themouse brain capillaries by RAP or a specificLRP1 antibody showed comparable reduction in Ab40 uptake andclearance suggesting RAP specific inhibition of LRP1 (Deane et al.,2004).
Transgenic AD mice models are widely used in AD research.Available studies showed that transgenic mice clear human Ab40from the brain at higher rate compared to human brain (Cirritoet al., 2003; Mawuenyega et al., 2010). Given the differences inclearance rate of Ab40 between mouse and human and the impor-tant contribution of BBB to Ab40 clearance, a set of experimentswere designed to compare Ab40 disposition in mouse and humanendothelial cells as BBB in vitro models.
Brain endothelial cells, astrocytes, pericytes, and neurons formthe neurovascular unit that contributes to Ab levels regulation in
Fig. 8. Schematic representation of Ab40 disposition in the brain. Different cellular compartmediates Ab40 transport across the BBB from the brain to the blood by transcytosis (1 and 2),toward the brain (4). In addition, endothelial cells express unknown components that mediatand astrocytes (6 and 7, respectively) mediates uptake and degradation of Ab40. In addition tby extracellular degradation (8). About 10e15% of Ab40 is cleared from interstitial fluid by
Please cite this article in press as: Qosa, H., et al., Differences in amyloidKinetic analysis and mechanistic modeling, Neuropharmacology (2014),
brain ISF (Bell and Zlokovic, 2009). While the astrocytes, pericytesand basement membrane play important role in regulating the BBBfunction, only the capillary endothelium forms the physical barrierseparating brain from blood. To cross the BBB toward the blood, Abutilizes specific transporters/receptors to facilitate its transportacross the endothelial physical barrier. Thus, in the current studywefocused our investigation on the specific contribution of endothelialcells to the clearance of Ab40 mediated by different transport pro-teins from brain to blood. Our preliminary studies demonstrated arapid depletion of Ab40 from bEnd3 cells to the media compared tohCMEC/D3. To explain this difference in Ab40 disposition, we char-acterized the activity of known Ab40 receptors and transporters inboth cells using uptake and transport studies. For degradationstudies, we used the percent of degraded Ab40 in the media as ameasure for cellular degradation due to the rapid release ofdegraded Ab40 fragments into themedia (Yamada et al., 2008). Cellsgrown on wells surface do not form polarized monolayers, thuscellular level of intact Ab40 is controlled by uptake by LRP1 andRAGE, efflux by P-gp, lysosomal degradation, and to a lesser extentby Ab degrading proteases. On the other hand, cells plated on atranswell filters are polarized and form tight junctions mimickingthe BBBwhereRAGE and P-gp are expressed on the apical sidewhileLRP1 is expressed on the basolateral side. In transport studies, theactivity of RAGE and P-gp was investigated in A / B direction, andactivity of LRP1 was investigated in B / A direction. Our findingsdemonstrated that in the uptake studies, LRP1 in bEnd3 andhCMEC/D3 mediates uptake of intact Ab40 and partial Ab40 degradation asconfirmed byRAP inhibition. On the other hand, LRP1 onlymediatestransport of intact Ab40 from B/ A in the transport studies, but notdegradation. This result is consistent with previous in vivo studiesdemonstrated LRP1 function in Ab40 transcytosis across the BBB(Castellano et al., 2012; Shibata et al., 2000), and is further supportedby in vitro transport studies conducted using primary brain endo-thelial cells plated on filters (Pflanzner et al., 2011). In addition, thisresult confirms our in vivo data where LRP1 mediates transport of
ments in the brain contribute to Ab40 clearance. Endothelial cells express LRP1 whichat the luminal side P-gp effluxes Ab40 to the blood (3), and RAGE mediates Ab40 uptakee uptake and degradation of Ab40 (5). LRP1 expressed at the cell membranes of neuronso cellular components, interstitial enzymes, IDE and NEP participate in Ab40 dispositionCSF bulk flow (9).
101102103104105106107108109110111112113114115116117118119120121122123124125126127128129130
-b clearance across mouse and human bloodebrain barrier models:http://dx.doi.org/10.1016/j.neuropharm.2014.01.023

H. Qosa et al. / Neuropharmacology xxx (2014) 1e1110
1234567891011121314151617181920212223242526272829303132333435363738394041424344454647484950515253545556575859606162636465
66676869707172737475767778798081828384858687888990919293949596979899
100101102103104105106107108109110111112113114115116117118119120121122123124125126127128129130
NP5381_proof ■ 25 January 2014 ■ 10/11
intact Ab40 across BBB. The partial reduction in Ab40 degradation byRAP observed in the uptake studies is possibly related to inhibitionof another receptor that play role in Ab40 degradation, most likelyexpressed at the apical side in the transport model, and on the cellsurface in the uptake model (Figs, 3 and 6C&D). Another possibleexplanation is based on previously reported studies, which utilizedLRP1minireceptors transfected into epithelial cells such as MDCK IIand CHO cells. These studies showed LRP1 to mediate Ab40 degra-dation mainly via endocytosis (Cam et al., 2005; Nazer et al., 2008).Conflicting findings regarding LRP1 function suggest LRP1mediatesboth Ab40 endocytosis and transcytosis probably depending on celltype and polarization state. This behavior of LRP1 is consistent withother members of the LDL receptors family shown previously tomediate endocytosis and transcytosis (Doherty and McMahon,2009; Tuma and Hubbard, 2003).
RAGE is a BBB receptor that is expressedmainly at the apical sideof brain endothelial cells and mediates influx of Ab40 from blood tobrain (Deane et al., 2003). In non-polarized and polarized bEnd3and hCMEC/D3 cells, RAGE has significant contribution to the up-take and A / B transport of intact Ab40 but not degradation. Theresults of current study support the finding of previous studiesreported role for RAGE in the transcytosis of Ab40 from blood tobrain (Candela et al., 2010; Deane et al., 2003).
P-gp, an efflux transporter, has been shown to contribute to Ab40clearance across BBB (Cirrito et al., 2005; Qosa et al., 2012; Tai et al.,2009). hCMEC/D3 cells demonstrated higher P-gp expression andactivity compared to bEnd3. While the effect of P-gp inhibition inbEnd3 cells was not obvious, this does not necessarily exclude thefunction of P-gp at the mouse BBB in Ab40 clearance. This findingcould be explained rather by the rapid uptake and degradation ofAb40 by bEnd3, which hinders the role of P-gp efflux. Findings fromour current and previous in vivo and in vitro P-gp inhibition studiessupported P-gp function at themouse BBB in the effluxof intact Ab40(Abuznait et al., 2013; Qosa et al., 2012), which entered the cells bytranscytosis via LRP1, and possibly other receptors, or Ab40 mole-cules leaked from cell organelles such as endosomes (HanssonPetersen et al., 2008). Though results from previous studiesobserved conflicting data about P-gp contribution to Ab40 clearanceacross BBB (Cirrito et al., 2005; Ito et al., 2006; Nazer et al., 2008; Taiet al., 2009), several imaging studies utilizing positron emissiontomography technology demonstrated an association between P-gpfunction and Ab plaque formation in AD brains (van Assema et al.,2012). Collectively, results of the current study and findings fromprevious mouse and human studies strongly suggest the contribu-tion of P-gp to the clearance of Ab40 across mouse and human BBB.
Ab40 transport proteins including LRP1, RAGE and P-gp, inaddition to degradation, have comparable functions in the mouseand human endothelial cells; however, differences in expressionand/or activity between the two species may explain differences inAb40 cellular disposition, thus, clearance rate. To identify the mainprocess(s) that contributes to such differences, a mechanistic two-compartment model was established. Parameters estimated by thismodel describe the activity of an overall process, i.e. overall uptake(include receptor mediated endocytosis and transcytosis), overallefflux (P-gp, other efflux proteins and exocytosis), and degradation,thus these parameters were termed as apparent parameters.Nevertheless, degradation of Ab40 described by this model repre-sents mainly lysosomal degradation and does not differentiatebetween different Ab40 metabolites. Consistent with observed data,the mechanistic model suggests higher Ab40 affinity to surfaceuptake receptors, and higher receptor activity in bEnd3 comparedto hCMEC/D3 cells. As demonstrated in the model scheme (Fig. 7A),removal of Ab40 from cellular compartment takes place by degra-dation and efflux. Apparent parameters obtained by mechanisticmodeling indicated the rate of Ab40 removal by efflux process was
Please cite this article in press as: Qosa, H., et al., Differences in amyloidKinetic analysis and mechanistic modeling, Neuropharmacology (2014),
similar in the two cells while degradation rate is higher in bEnd3compared to hCMEC/D3 cells (Table 1). The established model alsoshowed saturation of the uptake process in both cells was at lownanomolar concentrations of Ab40 (Km values of 19.8 and 49.1 nM inbEnd3 and hCMEC/D3 cells, respectively). Although these concen-trations are higher than those found in brains of normal individualsand in young transgenic mice (0.65 nM and 0.4 nM, respectively)(Brody et al., 2008; Kawarabayashi et al., 2001), they are muchlower than Ab40 concentrations in the ISF of AD patients and agedtransgenic mouse brains (2000 nM and 2500 nM, respectively)(Gregory and Halliday, 2005; Kawarabayashi et al., 2001). Thissuggests that in normal brains with low Ab40 concentration,clearance of Ab40 across BBB is efficient, while it is significantlydiminished in AD brains due to saturable clearance across the BBB.
Collectively, parameters estimated from the mechanistic modeland results obtained fromuptake and transport studies permit us toconclude that; i) the uptake process of Ab40 determines subsequentsteps including degradation and efflux processes, ii) brain endo-thelial cells mediate Ab40 degradation, however degradation rate isdependent on uptake rate which is higher in bEnd3, iii) both cellsmediate efficient efflux of intact Ab40 supporting the role of effluxtransporters as well as receptor mediated transcytosis in theclearance of Ab40 across endothelial cells.
Both, the in vitro and mechanistic models provided novel toolsto study Ab transport and clearance across BBB that should facili-tate our understanding of physiological and pathological factorsaffecting Ab clearance, however, it is worth noting that there aresome limitations with these models including; i) bEnd3 andhCMEC/D3 are immortalized cell lines, thus whether current find-ings can be translated to in vivo differences or not remain to beevaluated; ii) the transport kinetics of free Ab40 was evaluated here,however, in physiological and pathological conditions, Ab40 is alsofound in complex with chaperone proteins that influence itstransport kinetics; and iii) our studies focused on Ab40 dispositionand not Ab42. Compared to Ab40, Ab42 clearance across the BBB isslower (Ito et al., 2006), and prone to rapid aggregation. However,as both peptides use the same clearance pathways, the findings ofthis study with Ab40 are expected to be applicable for Ab42.
5. Conclusions
In conclusion, our results confirmed the significant contributionof BBB to the total clearance of Ab40 from brain compared toparenchymal degradation, and demonstrated that the higherclearance rate of Ab40 across the mouse BBB compared to humanBBB could be explained by the rapid uptake process precedes itsefflux and degradation. Furthermore, we successfully established amechanistic model that could predict Ab40 disposition in brainendothelial cells.
Conflict of interest
The authors declare no competing financial interest.
Acknowledgments
This research work was funded by an Institutional DevelopmentAward (IDeA) from the National Institute of General Medical Sci-ences of the National Institutes of Health under grant numberP20GM103424.
Appendix A. Supplementary data
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.neuropharm.2014.01.023.
-b clearance across mouse and human bloodebrain barrier models:http://dx.doi.org/10.1016/j.neuropharm.2014.01.023

Q2
Q1
H. Qosa et al. / Neuropharmacology xxx (2014) 1e11 11
1234567891011121314151617181920212223242526272829303132333435363738394041424344454647484950515253545556575859606162636465
66676869707172737475767778798081828384858687888990919293949596979899
100101102103104105106107108109110111112113114115116117118119120121122123124125126127128129
NP5381_proof ■ 25 January 2014 ■ 11/11
Uncited reference
Veinbergs et al., 2001.
References
Abuznait, A.H., Qosa, H., Busnena, B.A., El Sayed, K.A., Kaddoumi, A., 2013. Olive-oil-derived oleocanthal enhances beta-amyloid clearance as a potential neuro-protective mechanism against Alzheimer’s disease: in vitro and in vivo studies.ACS Chem. Neurosci..
Ballabh, P., Braun, A., Nedergaard, M., 2004. The blood-brain barrier: an overview:structure, regulation, and clinical implications. Neurobiol. Dis. 16, 1e13.
Banks, W.A., Robinson, S.M., Verma, S., Morley, J.E., 2003. Efflux of human andmouse amyloid beta proteins 1-40 and 1-42 from brain: impairment in a mousemodel of Alzheimer’s disease. Neuroscience 121, 487e492.
Barten, D.M., Guss, V.L., Corsa, J.A., Loo, A., Hansel, S.B., Zheng, M., Munoz, B.,Srinivasan, K., Wang, B., Robertson, B.J., Polson, C.T., Wang, J., Roberts, S.B.,Hendrick, J.P., Anderson, J.J., Loy, J.K., Denton, R., Verdoorn, T.A., Smith, D.W.,Felsenstein, K.M., 2005. Dynamics of {beta}-amyloid reductions in brain, cere-brospinal fluid, and plasma of {beta}-amyloid precursor protein transgenic micetreatedwitha {gamma}-secretase inhibitor. J. Pharmacol. Exp. Ther. 312, 635e643.
Basak, J.M., Verghese, P.B., Yoon, H., Kim, J., Holtzman, D.M., 2012. Low-density li-poprotein receptor represents an apolipoprotein E-independent pathway ofAbeta uptake and degradation by astrocytes. J. Biol. Chem. 287, 13959e13971.
Bell, R.D., Sagare, A.P., Friedman, A.E., Bedi, G.S., Holtzman, D.M., Deane, R.,Zlokovic, B.V., 2007. Transport pathways for clearance of human Alzheimer’samyloid beta-peptide and apolipoproteins E and J in the mouse central nervoussystem. J. Cereb. Blood Flow. Metab. 27, 909e918.
Bell, R.D., Zlokovic, B.V., 2009. Neurovascular mechanisms and blood-brain barrierdisorder in Alzheimer’s disease. Acta Neuropathol. 118, 103e113.
Brillault, J., Lam, T.I., Rutkowsky, J.M., Foroutan, S., O’Donnell, M.E., 2008. Hypoxiaeffects on cell volume and ion uptake of cerebral microvascular endothelialcells. Am. J. Physiol. Cell. Physiol. 294, C88eC96.
Brody, D.L., Magnoni, S., Schwetye, K.E., Spinner, M.L., Esparza, T.J., Stocchetti, N.,Zipfel, G.J., Holtzman, D.M., 2008. Amyloid-beta dynamics correlate withneurological status in the injured human brain. Science 321, 1221e1224.
Bu, G., 1998. Receptor-associated protein: a specialized chaperone and antagonistfor members of the LDL receptor gene family. Curr. Opin. Lipidol. 9, 149e155.
Cam, J.A., Zerbinatti, C.V., Li, Y., Bu, G., 2005. Rapid endocytosis of the low density li-poprotein receptor-related protein modulates cell surface distribution and pro-cessing of the beta-amyloid precursor protein. J. Biol. Chem. 280, 15464e15470.
Candela, P., Gosselet, F., Saint-Pol, J., Sevin, E., Boucau, M.C., Boulanger, E.,Cecchelli, R., Fenart, L., 2010. Apical-to-basolateral transport of amyloid-betapeptides through blood-brain barrier cells is mediated by the receptor foradvanced glycation end-products and is restricted by P-glycoprotein.J. Alzheimers Dis. 22, 849e859.
Castellano, J.M., Deane, R., Gottesdiener, A.J., Verghese, P.B., Stewart, F.R., West, T.,Paoletti, A.C., Kasper, T.R., DeMattos, R.B., Zlokovic, B.V., Holtzman, D.M., 2012.Low-density lipoprotein receptor overexpression enhances the rate of brain-to-blood Abeta clearance in a mouse model of beta-amyloidosis. Proc. Natl. Acad.Sci. U. S. A. 109, 15502e15507.
Cirrito, J.R., Deane, R., Fagan, A.M., Spinner, M.L., Parsadanian, M., Finn, M.B.,Jiang, H., Prior, J.L., Sagare, A., Bales, K.R., Paul, S.M., Zlokovic, B.V., Piwnica-Worms, D., Holtzman, D.M., 2005. P-glycoprotein deficiency at the blood-brainbarrier increases amyloid-beta deposition in an Alzheimer disease mousemodel. J. Clin. Investig. 115, 3285e3290.
Cirrito, J.R., May, P.C., O’Dell, M.A., Taylor, J.W., Parsadanian, M., Cramer, J.W.,Audia, J.E., Nissen, J.S., Bales, K.R., Paul, S.M., DeMattos, R.B., Holtzman, D.M.,2003. In vivo assessment of brain interstitial fluid with microdialysis revealsplaque-associated changes in amyloid-beta metabolism and half-life.J. Neurosci. 23, 8844e8853.
Citron, M., Oltersdorf, T., Haass, C., McConlogue, L., Hung, A.Y., Seubert, P., Vigo-Pelfrey, C., Lieberburg, I., Selkoe, D.J., 1992. Mutation of the beta-amyloid pre-cursor protein in familial Alzheimer’s disease increases beta-protein produc-tion. Nature 360, 672e674.
Deane, R., Bell, R.D., Sagare, A., Zlokovic, B.V., 2009. Clearance of amyloid-betapeptide across the blood-brain barrier: implication for therapies in Alz-heimer’s disease. CNS Neurol. Disord. Drug. Targets 8, 16e30.
Deane, R., Du Yan, S., Submamaryan, R.K., LaRue, B., Jovanovic, S., Hogg, E., Welch, D.,Manness, L., Lin, C., Yu, J., Zhu, H., Ghiso, J., Frangione, B., Stern, A.,Schmidt, A.M., Armstrong, D.L., Arnold, B., Liliensiek, B., Nawroth, P., Hofman, F.,Kindy, M., Stern, D., Zlokovic, B., 2003. RAGE mediates amyloid-beta peptidetransport across the blood-brain barrier and accumulation in brain. Nat. Med. 9,907e913.
Deane, R., Wu, Z., Sagare, A., Davis, J., Du Yan, S., Hamm, K., Xu, F., Parisi, M.,LaRue, B., Hu, H.W., Spijkers, P., Guo, H., Song, X., Lenting, P.J., VanNostrand, W.E., Zlokovic, B.V., 2004. LRP/amyloid beta-peptide interactionmediates differential brain efflux of Abeta isoforms. Neuron 43, 333e344.
Deane, R., Zlokovic, B.V., 2007. Role of the blood-brain barrier in the pathogenesis ofAlzheimer’s disease. Curr. Alzheimer Res. 4, 191e197.
Doherty, G.J., McMahon, H.T., 2009. Mechanisms of endocytosis. Annu Rev. Biochem78, 857e902.
Please cite this article in press as: Qosa, H., et al., Differences in amyloidKinetic analysis and mechanistic modeling, Neuropharmacology (2014),
Fuentealba, R.A., Liu, Q., Zhang, J., Kanekiyo, T., Hu, X., Lee, J.M., LaDu, M.J., Bu, G.,2010. Low-density lipoprotein receptor-related protein 1 (LRP1) mediatesneuronal Abeta42 uptake and lysosomal trafficking. PLoS One 5, e11884.
Gregory, G.C., Halliday, G.M., 2005. What is the dominant Abeta species in humanbrain tissue? A review. Neurotox. Res. 7, 29e41.
Hansson Petersen, C.A., Alikhani, N., Behbahani, H., Wiehager, B., Pavlov, P.F.,Alafuzoff, I., Leinonen, V., Ito, A., Winblad, B., Glaser, E., Ankarcrona, M., 2008.The amyloid beta-peptide is imported into mitochondria via the TOM importmachinery and localized to mitochondrial cristae. Proc. Natl. Acad. Sci. U. S. A.105, 13145e13150.
Ito, S., Ohtsuki, S., Terasaki, T., 2006. Functional characterization of the brain-to-blood efflux clearance of human amyloid-beta peptide (1-40) across the ratblood-brain barrier. Neurosci. Res. 56, 246e252.
Iwata, N., Tsubuki, S., Takaki, Y., Watanabe, K., Sekiguchi, M., Hosoki, E., Kawashima-Morishima, M., Lee, H.J., Hama, E., Sekine-Aizawa, Y., Saido, T.C., 2000. Identi-fication of the major Abeta1-42-degrading catabolic pathway in brain paren-chyma: suppression leads to biochemical and pathological deposition. Nat.Med. 6, 143e150.
Jan, A., Hartley, D.M., Lashuel, H.A., 2010. Preparation and characterization of toxicAbeta aggregates for structural and functional studies in Alzheimer’s diseaseresearch. Nat. Protoc. 5, 1186e1209.
Kandimalla, K.K., Curran, G.L., Holasek, S.S., Gilles, E.J., Wengenack, T.M.,Poduslo, J.F., 2005. Pharmacokinetic analysis of the blood-brain barrier trans-port of 125I-amyloid beta protein 40 in wild-type and Alzheimer’s diseasetransgenic mice (APP,PS1) and its implications for amyloid plaque formation.J. Pharmacol. Exp. Ther. 313, 1370e1378.
Kandimalla, K.K., Scott, O.G., Fulzele, S., Davidson, M.W., Poduslo, J.F., 2009. Mech-anism of neuronal versus endothelial cell uptake of Alzheimer’s disease amyloidbeta protein. PLoS One 4, e4627.
Kanekiyo, T., Liu, C.C., Shinohara, M., Li, J., Bu, G., 2012. LRP1 in brain vascularsmooth muscle cells mediates local clearance of Alzheimer’s amyloid-beta.J. Neurosci. 32, 16458e16465.
Kawarabayashi, T., Younkin, L.H., Saido, T.C., Shoji, M., Ashe, K.H., Younkin, S.G.,2001. Age-dependent changes in brain, CSF, and plasma amyloid (beta) proteinin the Tg2576 transgenic mouse model of Alzheimer’s disease. J. Neurosci. 21,372e381.
Mawuenyega, K.G., Sigurdson, W., Ovod, V., Munsell, L., Kasten, T., Morris, J.C.,Yarasheski, K.E., Bateman, R.J., 2010. Decreased clearance of CNS beta-amyloidin Alzheimer’s disease. Science 330, 1774.
Nazer, B., Hong, S., Selkoe, D.J., 2008. LRP promotes endocytosis and degradation,but not transcytosis, of the amyloid-beta peptide in a blood-brain barrierin vitro model. Neurobiol. Dis. 30, 94e102.
Pflanzner, T., Janko, M.C., Andre-Dohmen, B., Reuss, S., Weggen, S., Roebroek, A.J.,Kuhlmann, C.R., Pietrzik, C.U., 2011. LRP1 mediates bidirectional transcytosis ofamyloid-beta across the blood-brain barrier. Neurobiol. Aging 32, 2323 e2321e2311.
Poirier, A., Lave, T., Portmann, R., Brun, M.E., Senner, F., Kansy, M., Grimm, H.P.,Funk, C., 2008. Design, data analysis, and simulation of in vitro drug transportkinetic experiments using a mechanistic in vitro model. Drug Metab. Dispos. 36,2434e2444.
Qosa, H., Abuznait, A.H., Hill, R.A., Kaddoumi, A., 2012. Enhanced brain amyloid-betaclearance by rifampicin and caffeine as a possible protective mechanism againstAlzheimer’s disease. J. Alzheimers Dis. 31, 151e165.
Selkoe, D.J., 1993. Physiological production of the beta-amyloid protein and themechanism of Alzheimer’s disease. Trends Neurosci. 16, 403e409.
Shibata, M., Yamada, S., Kumar, S.R., Calero, M., Bading, J., Frangione, B.,Holtzman, D.M., Miller, C.A., Strickland, D.K., Ghiso, J., Zlokovic, B.V., 2000.Clearance of Alzheimer’s amyloid-beta(1-40) peptide from brain by LDLreceptor-related protein-1 at the blood-brain barrier. J. Clin. Investig. 106,1489e1499.
Silverberg, G.D., Mayo, M., Saul, T., Rubenstein, E., McGuire, D., 2003. Alzheimer’sdisease, normal-pressure hydrocephalus, and senescent changes in CSF circu-latory physiology: a hypothesis. Lancet Neurol. 2, 506e511.
Sommer, B., 2002. Alzheimer’s disease and the amyloid cascade hypothesis: tenyears on. Curr. Opin. Pharmacol. 2, 87e92.
Tai, L.M., Loughlin, A.J., Male, D.K., Romero, I.A., 2009. P-glycoprotein and breastcancer resistance protein restrict apical-to-basolateral permeability of humanbrain endothelium to amyloid-beta. J. Cereb. Blood Flow. Metab. 29, 1079e1083.
Tuma, P., Hubbard, A.L., 2003. Transcytosis: crossing cellular barriers. Physiol. Rev.83, 871e932.
van Assema, D.M., Lubberink, M., Rizzu, P., van Swieten, J.C., Schuit, R.C., Eriksson, J.,Scheltens, P., Koepp, M., Lammertsma, A.A., van Berckel, B.N., 2012. Blood-brainbarrier P-glycoprotein function in healthy subjects and Alzheimer’s diseasepatients: effect of polymorphisms in the ABCB1 gene. EJNMMI Res. 2, 57.
Veinbergs, I., Van Uden, E., Mallory, M., Alford, M., McGiffert, C., DeTeresa, R.,Orlando, R., Masliah, E., 2001. Role of apolipoprotein E receptors in regulatingthe differential in vivo neurotrophic effects of apolipoprotein E. Exp. Neurol.170, 15e26.
Yamada, K., Hashimoto, T., Yabuki, C., Nagae, Y., Tachikawa, M., Strickland, D.K.,Liu, Q., Bu, G., Basak, J.M., Holtzman, D.M., Ohtsuki, S., Terasaki, T., Iwatsubo, T.,2008. The low density lipoprotein receptor-related protein 1 mediates uptake ofamyloid beta peptides in an in vitro model of the blood-brain barrier cells.J. Biol. Chem. 283, 34554e34562.
130
-b clearance across mouse and human bloodebrain barrier models:http://dx.doi.org/10.1016/j.neuropharm.2014.01.023