Computational approach to dectect novel MCF-7 cell line inhibitors
-
Upload
simone-brogi -
Category
Technology
-
view
529 -
download
0
description
Transcript of Computational approach to dectect novel MCF-7 cell line inhibitors

Estrogen receptors (Estrogen receptors (ERER--α and ERα and ER--β subtypesβ subtypes) are members of a superfamily of ligand-activated transcription factors. Stimulation of estrogen receptors by endogenous estrogens plays an ) are members of a superfamily of ligand-activated transcription factors. Stimulation of estrogen receptors by endogenous estrogens plays an
important role in both male and female physiology. Estrogens are involved in the regulation of cholesterol and lipid levels, the skeletal system, the central nervous system, and reproductive important role in both male and female physiology. Estrogens are involved in the regulation of cholesterol and lipid levels, the skeletal system, the central nervous system, and reproductive
functions. However, estrogen stimulation is also implicated in the development of breast cancer. Consequently, many estrogen receptor ligands (SERMs: selective estrogen receptor modulators)functions. However, estrogen stimulation is also implicated in the development of breast cancer. Consequently, many estrogen receptor ligands (SERMs: selective estrogen receptor modulators)
are being developed with the aim of preventing estrogen mediated tumor growth. are being developed with the aim of preventing estrogen mediated tumor growth. MCF-7 cells are a well-characterized estrogen receptor (ER) positive control cell line and therefore are a useful MCF-7 cells are a well-characterized estrogen receptor (ER) positive control cell line and therefore are a useful
in vitro model to study the in vitro model to study the activity of new metabolites againstactivity of new metabolites against breast cancer breast cancer 11
Simone BrogiSimone Brogi and Andrea Tafiand Andrea Tafi
Dipartimento Farmaco Chimico Tecnologico, Università degli Studi di SienaDipartimento Farmaco Chimico Tecnologico, Università degli Studi di SienaVia Aldo Moro, I-53100 Siena, ItalyVia Aldo Moro, I-53100 Siena, Italy
Virtual screening is a powerful tool to discover new structures and Virtual screening is a powerful tool to discover new structures and
design new ligands of a biological target design new ligands of a biological target
In our study, the computational model PHERA was used to search In our study, the computational model PHERA was used to search
Asinex, Maybridge and NCI2000 chemical databases (about 500,000 Asinex, Maybridge and NCI2000 chemical databases (about 500,000
structurally diversified small molecules) for new chemical structures active structurally diversified small molecules) for new chemical structures active
against MCF-7 cell lineagainst MCF-7 cell line
Compounds with a fit cutoff value of 5 were selected by Compounds with a fit cutoff value of 5 were selected by CatalystCatalyst
software. Other filters were applied to identify entries against MCF-7 cell software. Other filters were applied to identify entries against MCF-7 cell
line: the compounds must satisfy the Lipiniski's rule of fiveline: the compounds must satisfy the Lipiniski's rule of five
The query identified 43 compounds. These molecules were considered The query identified 43 compounds. These molecules were considered
likely to be well-absorbed because satisfied Lipiniski's rule of fivelikely to be well-absorbed because satisfied Lipiniski's rule of five 33
These compounds were selected for docking analysisThese compounds were selected for docking analysis
43 molecules were selected, after virtual screening and docked with the 43 molecules were selected, after virtual screening and docked with the GOLDGOLD software, software,44 in the binding site (LBD) of ER- in the binding site (LBD) of ER-αα. For each compound several . For each compound several
scoring functions and a consensus scoring function were used to evaluate and rank the ER-scoring functions and a consensus scoring function were used to evaluate and rank the ER-α α ligand binding affinitiesligand binding affinities
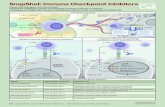
Compound 34, the most active molecule in training set, showed higher docking score and formed H-bonding with His-524 one active site residues of ER-Compound 34, the most active molecule in training set, showed higher docking score and formed H-bonding with His-524 one active site residues of ER-α α
(Fig.4a)(Fig.4a). In accordance, . In accordance, BrzozowskiBrzozowski and coworkers revealed that His-524 and Arg-394 are key residues in the active site (Fig.4d). and coworkers revealed that His-524 and Arg-394 are key residues in the active site (Fig.4d).55 Some of the hits retrieved Some of the hits retrieved
in database search, also showed good docking scores and formed similar type of interactions with these two active site amino acids (Fig.4b e 4c). The 12 in database search, also showed good docking scores and formed similar type of interactions with these two active site amino acids (Fig.4b e 4c). The 12
molecules which obtained a higher molecules which obtained a higher GOLDGOLD docking score were considered as final compounds and subjected to biological evaluation (Fig.5) docking score were considered as final compounds and subjected to biological evaluation (Fig.5)
Acknowledgment: We are grateful to Prof. Vassilios Roussis and co-workers for the chemical entities and the biological assayAcknowledgment: We are grateful to Prof. Vassilios Roussis and co-workers for the chemical entities and the biological assay
References: References: (1) Dowers, T. S (1) Dowers, T. S et al. J. Chem. Res. Toxicol.et al. J. Chem. Res. Toxicol. 2006, 2006, 1919, 1125; (2) , 1125; (2) Catalyst 4.08, Accelrys, Inc.: 9685 Scranton Road, San Diego, CA, USA; (3) Walters, W. P.; Catalyst 4.08, Accelrys, Inc.: 9685 Scranton Road, San Diego, CA, USA; (3) Walters, W. P.;
Murko, M. A. Murko, M. A. Adv. Drug Deliv. Rev.Adv. Drug Deliv. Rev. 2002, 2002, 5454, 255, 255; (4) Verdonk, M. ; (4) Verdonk, M. et al. J. Med. Chem. et al. J. Med. Chem. 2005, 2005, 4848, 6504; (5) , 6504; (5) Brzozowski, A. M. Brzozowski, A. M. et al.et al. Nature Nature 1997, 1997, 389, 389, 753753
>20012
>20011
188.410
170.69
148.58
124.37
67.46
60.15
58.54
40.93
31.82
26.41
Experimental Activity(µM)
Asinex Compounds
A new inclusive pharmacophore was generated for ER-α receptor, which estimated the inhibitory activity of ERMs with high accuracy (Catalyst A new inclusive pharmacophore was generated for ER-α receptor, which estimated the inhibitory activity of ERMs with high accuracy (Catalyst correlation correlation
factor of 0.91).factor of 0.91). Moreover, the Moreover, the interactionsinteractions necessary to bind ligands in the LBD necessary to bind ligands in the LBD werewere highlighted by PHERA at their proper position highlighted by PHERA at their proper positionss. We used the . We used the
pharmacophore model to perform virtual screening to discover new structures and design pharmacophore model to perform virtual screening to discover new structures and design MCF-7 cell lineMCF-7 cell line inhibitors inhibitors
After virtual screening 43 potential hits that showed good estimated activities as well as drug-like properties, were docked with the After virtual screening 43 potential hits that showed good estimated activities as well as drug-like properties, were docked with the GOLDGOLD software software
Compounds with higher Compounds with higher GOLDGOLD docking scores, that showed a binding mode very similar with experimentally proved compounds (Fig.4d), were chosen for docking scores, that showed a binding mode very similar with experimentally proved compounds (Fig.4d), were chosen for
biological evaluation against MCF-7 cell line giving interesting results (Fig.5)biological evaluation against MCF-7 cell line giving interesting results (Fig.5)
This outcome was obtained with a novel approach to generate the pharmacophore model and now we will work to optimize these potential lead This outcome was obtained with a novel approach to generate the pharmacophore model and now we will work to optimize these potential lead
compounds to increase activity against MCF-7 cell line. compounds to increase activity against MCF-7 cell line. These promising results encourage us to continue pursuing our target prioritization research These promising results encourage us to continue pursuing our target prioritization research
program. Expansion of this method to predict bioactivity on the basis of relationship between activities and chemical structures is expected to direct program. Expansion of this method to predict bioactivity on the basis of relationship between activities and chemical structures is expected to direct
compounds isolated in limited amounts towards targeted pharmacological testing, thereby accelerating the hit discovery processcompounds isolated in limited amounts towards targeted pharmacological testing, thereby accelerating the hit discovery process Fig. 5 Fig. 5 Biolocical evaluation of the Asinex compound Biolocical evaluation of the Asinex compound isolated after Virtual Screening and Molecular Dockingisolated after Virtual Screening and Molecular Docking
Fig. 1 Fig. 1 Superposition of PHERA and 34 (the most active compound in the training Superposition of PHERA and 34 (the most active compound in the training set). Pharmacophore features are color-coded: purple for hydrogen bond donor set). Pharmacophore features are color-coded: purple for hydrogen bond donor (HBD), green for hydrogen bond acceptor (HBA), sea green for hydrophobic (HBD), green for hydrogen bond acceptor (HBA), sea green for hydrophobic (HY1) and cyan for hydrophobic aromatic (HY2)(HY1) and cyan for hydrophobic aromatic (HY2)
Fig. 3 Fig. 3 Calculated versus observed value inhibitory Calculated versus observed value inhibitory
activity pICactivity pIC5050
Fig. 2 Fig. 2 SERM derivatives used in this study.SERM derivatives used in this study.
Fig. 4 Fig. 4 Molecular Docking: a) compound 34 (the most active compound in the training set); b) Asinex compound 1; c) Asinex compound 2; d) RaloxifeneMolecular Docking: a) compound 34 (the most active compound in the training set); b) Asinex compound 1; c) Asinex compound 2; d) Raloxifene
a b c d
Pharmacophore generationPharmacophore generation
Virtual screeningVirtual screening
Molecular DockingMolecular Docking
His-524
Arg-394
Calculated/Predicted value
Ob
se
rve
d v
alu
e
The The Catalyst/HypoRefineCatalyst/HypoRefine algorithm was used. algorithm was used.22 (which allows to generate hypotheses with (which allows to generate hypotheses with excluded volumes and thus accounting for steric excluded volumes and thus accounting for steric hindrance problems) (Fig.1)hindrance problems) (Fig.1)
The pharmacophore model for ERThe pharmacophore model for ER--αα (PHERA) was (PHERA) was generategenerate taking into account every class of SERMs taking into account every class of SERMs with significant structural diversity (Fig.2)with significant structural diversity (Fig.2)
The computational model was able to accurately The computational model was able to accurately estimate the activities of new chemical entities estimate the activities of new chemical entities (Fig.3)(Fig.3)
The interactions in the binding pocket of ERThe interactions in the binding pocket of ER--αα were were highlighted by PHERA at their proper positionhighlighted by PHERA at their proper position
Was used PHERA to perform a Virtual ScreeningWas used PHERA to perform a Virtual Screening
ConclusionConclusion
HBDHBD
HBAHBA
HY1HY1
HY2HY2HY2HY2
Arg-394Arg-394
Arg-394
His-524 His-524 His-524