Antioxidant activity and polyphenol content of endemic ...€¦ · Centaurea ragusina. L., a...
Transcript of Antioxidant activity and polyphenol content of endemic ...€¦ · Centaurea ragusina. L., a...
-
Material and methodsC. ragusina seeds were collected from their natural habitat near Dubrovnik. The sterilized seeds were germinated in containers filled with ½MS mediumcontaining 0.1 g L−1 myo-inositol, 0.1 mg L−1 thiamine × HCl, 0.5 mg L−1 pyridoxine × HCl, 0.5 mg L−1 nicotinic acid, 2.9 μM gibberellic acid (GA3), 0.5 μM 6-benzylaminopurine (BA), 30 g L−1 sucrose and 8 g L−1 agar [3]. The shoots isolated from the seedlings were first subcultured on the same compositionmedia. After 4 weeks in culture, shoots were transferred to the same composition media for callus initiation and to media containing 2.5 μM indole-3-butyric acid (IBA) for root initiation. Plant material (leaves and calli) were collected after 45 days of growth on media (½MS 2.9 μM GA3 + 0.5 μM BA and½MS 2.5 μM IBA) and after acclimatization from culture media (½MS 2.5 µM IBA). Leaves of plants collected from natural habitats (Katalinić brig - K andSustipan - S) were also tested. Plant material were lyophilized at -60°C and 0.01 mbar over 24h-period (Christ, Osterode am Harz, Germany). Three gramsof lyophilized leaves and calli were ground in a mortar and pestle with liquid nitrogen and homogenized in 60 mL of 80% ethanol. The samples were thensonicated for 15 mins at 25˚C and macerated for 72h at +4˚C. Following the period, the samples were additionally macerated at room temperature for a3-4h period with occasional stirring. Plant material was first filtered through Munktel filter discs grade 388 and then through GHP Acrodisc with 0.45 µmmembrane. Obtained extracts were evaporated by using a rotary evaporator, dissolved in water and sonicated for 15 minutes at 25˚C. The total phenol(TP) and flavonoid (TF) content was determined according to Zhishen et al. [4]. Total flavonols (TFL) and hydroxycinnamic acids (THA) were determinedusing methods reported by Howard et al. [5]. Proanthocyanidins (TPAN) were determinate using vanillin - HCl methods [6]. Antioxidant activity of plantextracts was determined according to the DPPH [7] and ABTS method [8]. Interactions of extracts with double stranded polynucleotides (poly A – poly Uand ctDNA) were evaluated by thermal denaturation experiments and CD spectroscopy [9].
References:1.Khammar A, Djeddi S. Pharmacological and Biological Properties of some Centaurea Species. Eur J Sci Res 2012; 84: 398-416.2.Radić S, Peharec Štefanić P, Lepeduš H, Roje V, Pevalek-Kozlina B. Salt tolerance of Centaurea ragusina L. is associated with efficient osmotic adjustment and increased antioxidative capacity. Environ Exp Bot 2013; 87: 39-48.3.Pevalek-Kozlina B. In vitro propagation of Centaurea ragusina L., a Croatian endemic species. Acta Biol Cracov Ser Bot 1998; 40: 21-24.4.Zhishen J, Mengcheng T, Jianming W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem 1999; 64: 555-559.5.Howard LR, Clark JR, Brownmiller C. Antioxidant capacity and phenolic content in blueberries as affected by genotype and growing season. J Sci Food Agric 2003; 83: 1238-1247.6.Sun B, da-Silva JMR, Spranger I. Critical factors of vanillin assay for catechins and proanthocyanidins. J Agric Food Chem 1998; 46: 4267-4274.7.Germano MP, Pasquale RD, D’Angelo V, Catania S, Silvari V, Costa C. Evaluation of extracts and isolated fraction from Capparis spinosa L. buds as an antioxidant source. J Agric Food Chem 2002; 50: 1168–1171.8.Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med 1999; 26: 1231–1237.9. Tumir L-M, Radić Stojković M, Piantanida I. Come-back of phenanthridine and phenanthridinium derivatives in 21st century. Beilstein J Org Chem 2014; 10: 2930-2954.
Antioxidant activity and polyphenol content of endemic plant species Centaurea ragusina L.
Valerija Vujčić1, Sandra Radić Brkanac1, Marijana Radić Stojković2, Mirko Ruščić3, Branka Pevalek-Kozlina1
1Division of Botany, Department of Biology, Faculty of Science, University of Zagreb, Rooseveltov trg 6, HR-10000 Zagreb, Croatia ([email protected], [email protected], [email protected]) 2Division of Organic Chemistry and Biochemistry, Rudjer Boskovic Institute, Bijenička c. 54, HR-10000 Zagreb, Croatia ([email protected])
3Department of Biology, Faculty of Science, University of Split, Teslina 12, HR-21000 Split, Croatia ([email protected])
TP TF TFL THA TPAN DPPH ABTS
Leaf - IBA/acclimatization 50.0±0.04b 33.9±0.09b 21.9±0.86b 51.9±2.25a 12.3±0.03a 80.9±0.03e 99.6±0.13a
Leaf - IBA 21.0±2.44f 7.5±2.73d 11.5±0.13e 21.6±0.42e 8.7±0.05c 92.1±0.10abc 79.3±0.29b
Leaf - BA + GA3 23.9±1.19e 9.4±1.11d 11.4±0.13e 23.0±0.48e 10.1±0.08b 90.3±0.23c 77.4±0.65b
Callus - BA + GA3 55.7±2.58a 54.4±6.12a 24.1±0.94a 35.5±1.46b 8.3±0.02d 89.5±0.11c 98.6±0.09a
Leaf - S 32.4±0.42cd 18.5±0.30c 17.0±0.41c 31.9±0.81c 1.5±0.03g 93.5±0.27ab 99.6±0.02a
Leaf - K 29.5±0.43d 20.4±0.94c 13.3±0.46d 27.0±0.92d 1.9±0.06e 84.4±1.97d 61.6±6.54c
Leaf - S + K 31.6±0.82cd 20.2±0.43c 16.0±0.97c 30.5±1.59c 1.7±0.01f 90.4±1.71bc 99.7±0.17a
GA 95.7±0.11 100.4±0.16
Table 1. Polyphenol content: total phenols (TP; mg GAE/g DW), total flavonoids (TF; mg QE/g DW), total flavonols (TFL; mg QE/g DW),total hydroxycinnamic acid (THA; mg CAE/g DW), total proanthocyanidins (TPAN; mg CE/g DW) and antioxidant activity measured byDPPH (DPPH; % inhibition) and ABTS (ABTS; % inhibition) in endemic Croatian plant species C. ragusina L.
Values represent mean ± standard deviation of 3 replicates. Different letters indicate significant difference at p < 0.05.
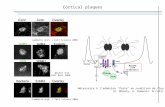
Figure 1. CD spectrum A) of ethanol/aqueous extracts ofleaves after acclimatization (80 g L-1), CD spectrum andΔTm values upon titration with B) poly A - poly U (c = 3 ×10-5 mol dm-3) and C) ctDNA (c = 3 × 10-5 mol dm-3) insodium cacodylate buffer (pH 7, I = 0.05 mol dm-3).
Table and figure legend:GAE - gallic acid equivalent, QE - quercetin equivalent, CAE - caffeic acid equivalent, CE - catechin equivalentLeaf - IBA/acclimatization - in vivo ethanol aqueous extract of leaves of C. ragusina (extracted in 80% ethanol, evaporated and dissolved in water to final concentration of 20 g L-1) - after acclimatization from culture media (½MS 2.5 µM IBA)Leaf - IBA - in vitro ethanol aqueous extract of leaves of C. ragusina (extracted in 80% ethanol, evaporated and dissolved in water to final concentration of 20 g L-1) - after 45 days of growth on media containing ½MS 2.5µM IBALeaf - BA + GA3 - in vitro ethanol aqueous extract of leaves of C. ragusina (extracted in 80% ethanol, evaporated and dissolved in water to final concentration of 20 g L-1) - after 45 days of growth on media containing ½MS 2.9 µM GA3 + 0.5 µM BACallus - BA + GA3 - in vitro ethanol aqueous extract of calli of C. ragusina (extracted in 80% ethanol, evaporated and dissolved in water to final concentration of 20 g L-1) - after 45 days of growth on media containing ½MS 2.9 µM GA3 + 0.5 µM BALeaf - S - in vitro ethanol aqueous extract of leaves of C. ragusina (extracted in 80% ethanol, evaporated and dissolved in water to final concentration of 20 g L-1) - collected at natural habitat (Split: Sustipan)Leaf - K - in vitro ethanol aqueous extract of leaves of C. ragusina (extracted in 80% ethanol, evaporated and dissolved in water to final concentration of 20 g L-1) - collected at natural habitat (Split: Katalinić brig)Leaf - S + K - in vitro ethanol aqueous extract of leaves of C. ragusina (extracted in 80% ethanol, evaporated and dissolved in water to final concentration of 20 g L-1) - collected at natural habitats (Split: Katalinić brig and Sustipan)GA - gallic acid (concentration of 20 g L-1)
Introduction The genus of Centaurea (Asteraceae) represents an attractive source of bioactivesubstances [1]. Centaurea ragusina L. is an endemic Croatian plant species growing in thegapes of vertical limestone cliffs along the coast and on some islands of the Adriatic Sea.On its native habitat it is affected not only by drought and high temperatures, but to someextent, salinity as well. Accordingly, it has been described as a halophyte with certainxeromorphic characteristics [2]. Like many species from Centaurea genus, C. ragusina isalso interesting as a potential source of different bioactive substances such as flavonoidsand polyphenols that could be used for medicinal purposes. The goal of this work is todetermine polyphenol content and antioxidant activity in C. ragusina L. cultivated in vitro(½MS 2.9 μM GA3 + 0.5 μM BA and ½MS 2.5 μM IBA) and collected at two natural habitatsin Split (Katalinić brig - K and Sustipan - S). To clarify biological activity of C. ragusina,interactions of extracts with double stranded polynucleotides (poly A – poly U and ctDNA)were also studied.
Conclusions The results obtained suggest that the plant species C. ragusina grown in vitro can be efficiently used as a potential source of polyphenols and antioxidants in food, pharmacological and cosmetics industry as it is equally rich with phytochemicals as its wild grown counterparts.
Acknowledgments This study has been co-financed by the European Union: the European Social Fund as part of the human Resources Development 2007 - 2013, as part of Project "HR.3.2.01-0290 Biological and phytochemical activity of Centaurea ragusina L. (BioFitoCen)“. http://www.biofitocen.biol.pmf.hr
ΔTm= 18.36
ΔTm= 5.75
mailto:[email protected]:[email protected]:[email protected]:[email protected]:[email protected]://europa.eu/http://europa.eu/http://www.biofitocen.biol.pmf.hr/
Acknowledgments �This study has been co-financed by the European Union: the European Social Fund as part of the human Resources Development 2007 - 2013, as part of Project "HR.3.2.01-0290 Biological and phytochemical activity of Centaurea ragusina L. (BioFitoCen)“. �http://www.biofitocen.biol.pmf.hr �









![Author Manuscript NIH Public Access ‡, May L. Lam ... J Biol Chem.pdf · Ci/mmol) and [3H]CGP12177 (specific activity = 51 Ci/mmol) were from AmershamBiosciences. All other materials](https://static.fdocument.org/doc/165x107/5f9a3bd75b96fb195c761951/author-manuscript-nih-public-access-a-may-l-lam-j-biol-chempdf-cimmol.jpg)