α-Tocopherol induces proatherogenic changes to HDL2 & HDL3: An in vitro and ex vivo investigation
Transcript of α-Tocopherol induces proatherogenic changes to HDL2 & HDL3: An in vitro and ex vivo investigation
at SciVerse ScienceDirect
Atherosclerosis 226 (2013) 392e397
Contents lists available
Atherosclerosis
journal homepage: www.elsevier .com/locate/atherosclerosis
a-Tocopherol induces proatherogenic changes to HDL2 & HDL3: Anin vitro and ex vivo investigation
Lauren Wade, Nida Nadeem, Ian S. Young, Jayne V. Woodside, Ann McGinty,Cyril McMaster, Jane McEneny*
Nutrition and Metabolism Group, Centre for Public Health, Queen’s University Belfast, Pathology Building, Grosvenor Road, Belfast BT12 6BJ, UK
a r t i c l e i n f o
Article history:Received 29 June 2012Received in revised form24 September 2012Accepted 15 November 2012Available online 19 December 2012
Keywords:a-TocopherolHDL subfractionsOxidationPON-1 and LCAT activities
* Corresponding author. Tel.: þ44 28 90632517; faxE-mail address: [email protected] (J. McEneny
0021-9150/$ e see front matter � 2012 Elsevier Irelahttp://dx.doi.org/10.1016/j.atherosclerosis.2012.11.032
a b s t r a c t
Introduction: High density lipoproteins (HDL) have considerable potential for improving cardiovascularhealth. Additionally, epidemiological studies have identified an inverse relationship between a-tocopherolintake and cardiovascular disease, which has not been translated in randomised controlled trials.Objectives: This study assessed if increased a-tocopherol within HDL2 and HDL3 (HDL2&3) influenced theirantiatherogenic potential. In the first of two in vitro investigations, the oxidation potential of HDL2&3 wasassessed when a-tocopherol was added following their isolation. In the second, their oxidation potentialwas assessed when HDL2&3 were isolated from serum pre-incubated with a-tocopherol. Additionally,a 6-week placebo-controlled intervention with a-tocopherol assessed if a-tocopherol influenced theoxidation potential and activities of HDL2&3-associated enzymes, paraoxonase-1 (PON-1) and lecithincholesteryl acyltransferase (LCAT).Results: Conflicting results arose from the in vitro investigations, whereby increasing concentrations ofa-tocopherol protected HDL2&3 against oxidation in the post-incubated HDL2&3, and promoted HDL2&3-oxidation when they were isolated from serum pre-incubated with a-tocopherol. Following the 6-weekplacebo-controlled investigation, a-tocopherol increased in HDL2&3, while HDL2&3 became moresusceptible to oxidation, additionally the activities of HDL2&3-PON-1 and HDL2-LCAT decreased.Conclusion: These results have shown for the first time that a-tocopherol induces changes to HDL2&3,which could contribute to the pathophysiology of cardiovascular disease.
� 2012 Elsevier Ireland Ltd. All rights reserved.
1. Introduction
Cardiovascular disease (CVD) constitutes a major health concernin industrialised nations [1] and significant research has high-lighted the role played by LDL in enhancing its development [2].However, more recently the focus of research has shifted towardsHDL, as this lipoprotein has considerable potential for improvingcardiovascular health [3]. In addition, numerous studies haveidentified that antioxidants have the potential to reduce CVD [4,5],due to their ability to decrease the oxidative stress associated withits development [6]. Therefore, in relation to this it has been sug-gested that dietary strategies, to include antioxidant supplements,may influence the cardioprotective properties of HDL, particularlyby affecting its oxidative potential [7]. For this reason it is importantto understand the effects of such antioxidants and their ascribedcardioprotective properties towards HDL. One such antioxidant,
: þ44 28 90235900.).
nd Ltd. All rights reserved.
which may influence HDL’s oxidant potential and function isa-tocopherol, themajor form of vitamin E and themost potent lipidsoluble antioxidant in the human diet [8].
In vitro models have demonstrated that at sufficient dosesa-tocopherol has the ability to (i) reduce the oxidative modificationof LDL, (ii) decrease LDL deposition in arterial walls, (iii) reducemonocyte adhesion onto the endothelium, and (iv) inhibit plateletactivation [9]. Therefore, these findings have fuelled numerousstudies investigating the role of a-tocopherol in the prevention andtreatment of atherosclerosis. Despite the promising experimentaland epidemiological data indicating an inverse relationship betweena-tocopherol intake and CVD [10,11], numerous studies have failedto support these findings, producing conflicting results. For example,several studies have indicated that a-tocopherol supplementationhas a beneficial effect towards preventing CVD [12], while othershave indicated a neutral effect [12], and others a deleterious effect[13]. In addition, the study of Nadeem et al. [5] reported thata-tocopherol was detrimental to the peroxidation of total-HDLwhen isolated from serum pre-incubated with a-tocopherol, or
L. Wade et al. / Atherosclerosis 226 (2013) 392e397 393
following an ex vivo supplementation with g-tocopherol. This pro-oxidant effect was also in support of previous findings, wherea-tocopherolwas shown to be detrimental to the oxidation potentialof total-HDL when isolated from pre-incubated serum [14].
Therefore, a-tocopherol may be producing pro-inflammatory ordysfunctional HDL, which several lines of evidence suggest, contrib-utes to the pathophysiology of CVD. As a result this study examinedother aspects of HDL function, to include its association with serumamyloidA (SAA) and the functionof severalHDL-associatedenzymes,namely, paroxonase-1 (PON-1), cholesteryl ester transfer protein(CETP) and lecithin cholesteryl acyltransferase (LCAT).
Ample evidence indicates that SAA related inflammation inter-feres with the functioning of HDL particles [15], as this associationreduces HDL’s ability to participate in reverse cholesterol transport(RCT). Additionally, PON-1, whose principle role is to prevent theoxidativemodification of apo B containing lipoproteins, particularlyLDL [16], has been shown to be less effective in dysfunctional HDL[17]. Furthermore, although CETP is essential for the normal func-tioning of HDL, a high activity may be detrimental to HDL remod-elling [3], which may be the case in dysfunctional HDL [18]. Finally,LCAT is essential for HDL metabolism, and also possesses antioxi-dant properties [3]; however, its role in dysfunctional HDL is lesswell defined.
Moreover, as several in vitro studies [5,14] have indicated thata-tocopherol may produce detrimental changes to the oxidationpotential of HDL, and as clinical interventions with a-tocopherolhave been mainly inconclusive in demonstrating a cardioprotectiveeffect, this study was implemented to investigate potential mech-anisms underlying this disparity by examining the two majorsubfractions of HDL, namely HDL2 and HDL3 (HDL2&3).
2. Material and methods
This work was approved by the Research Ethics Committee,School of Medicine, Dentistry and Biomedical Sciences, Queen’sUniversity, Belfast (ethics number 10/05) and informed writtenconsent was received by all participants.
In vitro investigations e Fasting peripheral venous blood wasobtained fromnormal, healthy volunteers with no known history ofCVD and not taking dietary supplements known to affect theparameters being measured. Blood was collected into serum clotactivator tubes. These were then subjected to low speed centrifu-gation at 1100� gmax for 20 min at 4 �C, in a Beckman J-6Bcentrifuge. Serum was harvested, pooled and separated into twoaliquots, one was pre-incubated with 20 mL ethanol/mL (control),and the second was pre-incubated with an equivalent volume ofincreasing concentrations of a-tocopherol (0, 0.5. 1.0, 2.5, 5.0 or10 mM, final concentrations), which was dissolved in ethanol.Samples were incubated for 2 h at 37 �C; these were separated intoaliquots and frozen at �70 �C for the following analyses.
(N.B. We have demonstrated that ethanol does not influence theintegrity/properties of the isolated lipoproteins, results not shown).
HDL2&3 isolation and oxidation e HDL2&3 were isolated fromserum by rapid flotation ultracentrifugation [19], which wasa 3 � 2 h procedure. The oxidation potential and the incorporationof a-tocopherol into HDL2&3 were then assessed.
Oxidation: The protein concentration of HDL2&3 was assessedusing the Coomassie blue reaction with proteins, this allowed eachsubfraction to be standardised to 50 mg/mL. Oxidation was medi-ated by the addition of a copper (II) chloride solution (CuCl2), finalconcentration 5 mM [19].
Two oxidation assessments were performed. In the first, HDL2&3were isolated from control serum (containing 20 mL ethanol/mLserum) and these were oxidised in the presence of increasingconcentrations of a-tocopherol (0, 0.5. 1.0, 2.5, 5.0 or 10 mM, final
concentrations), with the antioxidant being added in a volume of20 mL/mL of lipoprotein. In the second, the oxidation potential ofHDL2&3 was assessed when they were isolated from serum that hadbeen pre-incubated with increasing concentrations of a-tocopherol(0, 0.5. 1.0, 2.5, 5.0 or 10 mM, final concentrations, as described abovethese had been added in a volume of 20 mL a-tocopherol/mL serum).For both in vitro scenarios, the oxidation process was monitored ina thermostatically controlled UV spectrophotometer (SpectraMax190, Molecular Devices Inc. USA) at 37 �C. Absorbance change wasmeasured at 234 nm and results expressed as the kinetic parametertime at half maximum (time1/2max), an equivalent of lag time.
Incorporationofa-tocopherole The concentration of a-tocopherolwas measured in HDL2&3 isolated from the pre-incubated serum byhigh performance liquid chromatography (HPLC) with diode arraydetection [20]. a-Tocopherol was expressed as nmol/mg protein inHDL2&3.
2.1. Ex vivo investigation
Study population and design e This study was a randomiseddouble-blind placebo-controlled trial carried out on 30 healthyvolunteers, mainly recruited from the staff and students fromQueen’s University, Belfast. Volunteers were excluded if they wereconsuming antioxidant supplements or medication known to affectthe variables being tested. Additionally, volunteers were asked tomaintain their normal diet throughout the study period. Thevolunteers were randomised using computer-generated randomnumbers and assigned to receive either placebo or 400 IU ofa-tocopherol daily, for 6 weeks (both placebo and a-tocopherolwere a kind gift from Cultech, Biospeciality Products, Port Talbot,West Glamorgan, Wales). Fasting blood was obtained at thebeginning (week-0) and end of the intervention period (week-6).Serum was isolated as described above and the following analyseswere performed.
HDL2&3 isolation and oxidation e HDL2&3 were isolated andoxidised as described above for pre-incubated serum.
a-Tocopherole The concentration of a-tocopherol wasmeasuredin serum and in HDL2&3 by HPLC, as described above for pre-incubated serum. a-Tocopherol was expressed as mM in serumand as nmol/mg protein in HDL2&3.
Apolipoprotein AI (Apo AI) e The single radial immunodiffusiontechnique described by McPherson et al. [19] was utilised todetermine apo AI concentration in HDL2&3. Apo AI concentrationwas expressed as mg/L.
SAA e SAAwas measured in serum and in HDL2&3 by an enzymelinked immunosorbent assay (ELISA), as per manufacturer’sinstructions (Invitrogen Human SAA kit, KHA0011). Analysis wasperformed using a Grifols TRITURUS system (Italy). SAA wasexpressed as mg/L in serum and as mg/mg protein in HDL2&3.
PON-1 e Paraoxonase arylesterase activity was measured bymonitoring the hydrolysis of phenylacetate, by amodification of themethod described by Hasselwander et al. [21]. In brief, serum (5 mL),HDL2 (200 mL) or HDL3 (20 mL) were adjusted to a total volume of2700 mL with the assay buffer (Tris HCl, 20 mM, pH 8.0, containing1.0 mM CaCl2) followed by the addition of 300 mL of 10 mM phe-nylacetate. The production of phenol was monitored spectropho-tometrically at 270 nm in a UV plate reader at 10 s intervals. Changein absorbance (DA) was recorded at time ¼ 3 min. This was con-verted to arylesterase activity (U/L) by the following equation:
ArylesteraseðU=LÞ ¼ DA� Vt
T � ε� Vs� 103
where DA is change in absorbance; Vt is total volume of sample(3.00 mL); T is time in min; ε is phenol molar extinction coefficient
Post-incubated HDL2&3
0.0 2.5 5.0 7.5 10.0
25
75
125
175HDL
2
HDL3
-tocopherol added to HDL2&3
( mol/L)
Tim
e1
/2
m
ax.
(m
in
utes)
*
*
μα
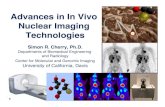
Fig. 1. Post-incubated HDL2&3: HDL2&3 were isolated from serum not pre-incubatedwith a-tocopherol. The two subfractions were oxidised by CuCl2 (5 mM, final concen-tration) in the presence of a-tocopherol (0, 0.5, 1.0, 2.5, 5 or 10 mM). The oxidationprocess was monitored in a thermostatically controlled spectrophotometer (37 �C).Absorbance change was measured at 234 nm and results expressed as the kineticparameter time1/2max (min). Results are mean (SEM), n ¼ 9 per concentration. *Trendsacross the groups were determined by one-way ANOVA.
L. Wade et al. / Atherosclerosis 226 (2013) 392e397394
(1.310 M�1 cm�1); Vs is sample volume in mL (0.005 mL serum,0.2 mL HDL2, 0.02 mL HDL3).
One unit of arylesterase activity (U) was defined as 1 mmol ofphenol generated per minute. PON-1 activity was expressed as U/Lin serum and as U/mg protein in HDL2&3.
2.2. CETP and LCAT
CETP activity was measured in serum and HDL2&3 usinga commercially available fluorimetric activity assay (Roar Biomed-ical, NY, USA; CETP Activity Assay Kit, RB-RPAK), as per manufac-turer’s instructions.
In brief, a standard curve was prepared by taking 5 mL of donor(0.005 mL � 260 nmol/mL) added to isopropanol (2 mL), this wasthen serially diluted to give 130, 65, 32.5, 16.3, 8.1 and 4.1 pmol/mL.For analysis the assay buffer was reconstituted with distilled water(5 mL assay buffer plus 45 mL H2O), to yield 50 mL of 1� assaybuffer (150 nM NaCl, 10 mM Tris, 2 mM EDTA, pH 7.4). Then induplicate, 5 mL of the study sample or standard, 4 mL donor, 4 mLacceptor and 187 mL of assay buffer were added to a black 96 ewellfluorimetric plate (Costar 28906040), a blank solution was alsoprepared by omitting sample (4 mL donor, 4 mL acceptor and 192 mLbuffer). The plate was then incubated in a water bath at 37 �C for3 h. Following incubation the increase in fluorescence, whichrepresents the rate of cholesteryl ester transfer from the syntheticdonor particles to the acceptor molecule, was measured usinga Tecan Genios Fluorimeter (Ex 465 nm; Em 535 nm). The fluores-cence intensity was determined by subtracting the blank fluores-cence intensity from each sample and reads against the standardcurve. CETP activity was expressed as mmol transferred per 3 h inserum and as mmol transferred per 3 h/mg protein in HDL2&3.
LCAT activity was measured in serum and HDL2&3 usinga commercially available fluorimetric activity assay (Roar Biomed-ical, NY, USA; LCAT Activity Assay Kit, RB-LCAT), as per manufac-turer’s instructions.
In brief, two assay buffer were prepared, assay buffer I (150 mMNaCl, 10 mM Tris, 1 mM EDTA, 4 mM 2-mercaptoethanol, pH 7.4)and assay buffer II (same as above, but omitting mercaptoethanol).The READ reagent provided (1 mL) was then reconstituted with99 mL of LCAT assay buffer II. Then, 5 mL of study sample (serum,HDL2 or HDL3), plus 1 mL of LCAT substrate (phosphatidylcholine)and 194 mL of LCAT buffer I, were added to a 2 mL O-ring tube andincubated in a water bath at 37 �C, for a period of 4 h. Followingincubation, 67 mL of the incubated mixture, plus 200 mL of dilutedREAD reagent were added in duplicated to wells of a black 96-wellfluorometric plate. The fluorescence was then read at differentwavelengths (1) Ex 340 nm, Em 390 nm and (2) Ex 340 nm and Em470 nm, again using a Tecan Genios Fluorimeter. The emissionintensities of 390 nm and 470 nm represent the emission of thesubstrate hydrolysed (390 nm) and not hydrolysed (470 nm).During hydrolysis by LCAT, the monomer emission at 390 nmincreases, indicating increased LCAT activity. LCAT activity wasexpressed as its ratio in serum and as its ratio/mg protein inHDL2&3.
Statistics e Statistics were completed using the statisticalpackage SPSS, version 17. Normally distributed baseline data wasassessed for n> 2 groups or time points by a one-way ANOVA or bythe KruskaleWallis test when not normally distributed. Differencesin changes between n ¼ 2 independent groups, where data werenot normally distributed were analysed by the ManneWhitney Utest. Pre vs. post comparisons were analysed by the Wilcoxonsigned rank test. Correlations between the incorporation ofa-tocopherol into HDL2&3 and change in time1/2max of HDL2&3 wereassessed using Pearson’s correlation coefficient. All variables weresummarised as mean (SEM) and significance was set as p � 0.05.
3. Results
3.1. In vitro investigations
This investigation determined if a-tocopherol influenced theoxidation potential of HDL2&3 when added to these subfractionsfollowing their isolation from serum (post-incubated HDL) or whenadded to serum before the isolation of HDL2&3 (pre-incubatedserum).
Post-incubate HDL2&3 (Fig. 1): Compared to time1/2max for zeroaddition of a-tocopherol, results identified that both subfractionswere protected against oxidation as a-tocopherol increased(p < 0.001, trends across the concentrations for both subfractions).Furthermore, a positive correlation was identified between thisadded a-tocopherol and time1/2max (HDL2, r ¼ 0.962, p < 0.001;HDL3, r ¼ 0.918, p < 0.001).
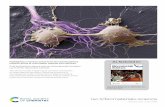
Pre-incubatedserum(Fig. 2a&b):WhenHDL2&3were isolated fromserum pre-incubated with a-tocopherol, although the a-tocopherolincreased within the subfractions (p < 0.001, trends across theconcentrations for both subfractions), both subfractions were morereadily oxidised (p < 0.001, trends across the concentrations for bothsubfractions), comparedtozeroadditionofa-tocopherol.Additionally,a negative correlation was identified between the a-tocopherolincorporation and time1/2max (HDL2, r ¼ �0.843, p < 0.001; HDL3,r ¼ �0.841, p < 0.001).
3.2. Ex vivo investigation
This study investigated the role of a placebo-controlled 6-weekintervention with a-tocopherol on the susceptibility of HDL2&3 tooxidation, together with functional aspects of HDL2&3-associatedproteins namely, SAA, apo AI, PON-1, CETP and LCAT.
3.3. Subject characteristics
Subject characteristics were comparable between the placeboand a-tocopherol groups. Both consisted of 6 males and 9 femalesand in the placebo vs. a-tocopherol comparisons, mean age (years)was 32.7 (0.84) vs. 37.7 (0.83), p¼ 0.122, andmean BMI (kg/m2) was23.4 (0.21) vs. 24.1 (0.22), p ¼ 0.699.
Pre-incubated Serum (HDL2)
0.0 2.5 5.0 7.5 10.0
30
35
40
45
50
3.50
3.75
4.00
4.25
4.50
-tocopherol added to serum
( mol/L)
Tim
e1/2 m
ax.
(m
in
utes)
-T
oco
ph
ero
l
(m
ol/m
g p
ro
tein
)
Pre-incubated Serum (HDL3)
0.0 2.5 5.0 7.5 10.0
30
35
40
45
50
7.75
8.25
8.75
9.25
-tocopherol added to serum
( mol/L)
Tim
e1/2 m
ax.
(m
in
utes)
-T
oco
ph
ero
l
(m
ol/m
g p
ro
tein
)
*
*
*
*
a
b
αμ
μα
ααμ
μ
Fig. 2. a&b. Pre-incubate serum: HDL2&3 were isolated from serum pre-incubated witha-tocopherol (0, 0.5, 1.0, 2.5, 5 or 10 mM). The incorporation of a-tocopherol intoHDL2&3 was assessed by HPLC with diode array detection [20] and expressed as mmol/mg protein. The two subfractions were oxidised by CuCl2 (5 mM, final concentration).The oxidation process was monitored in a thermostatically controlled spectropho-tometer (37 �C) by following the production of conjugated dienes at 234 nm. Resultsare expressed as the kinetic parameter time1/2max (min). *Trends across the groupswere determined by one-way ANOVA. ∎ Time1/2max; D a-Tocopherol incorporation.Results are mean (SEM): n ¼ 9 per concentration.
Table 1Ex vivo: a-tocopherol and time1/2max for serum and HDL2&3.
Group Baseline Week-6 % Change pa pb
a-TocopherolSerum Placebo 33.20 (2.12) 31.28 (2.37) �6.13 (2.71) 0.124 0.000
Tocopherol 33.89 (1.53) 42.21 (2.12) 24.38 (3.06) 0.001HDL2 Placebo 3.71 (0.29) 3.66 (0.31) �0.22 (5.99) 0.485 0.002
Tocopherol 3.58 (0.39) 4.33 (0.56) 22.29 (5.00) 0.004HDL3 Placebo 8.07 (0.35) 7.93 (0.39) �1.33 (3.43) 0.695 0.026
Tocopherol 7.69 (0.61) 9.08 (1.07) 18.94 (6.89) 0.023Time1/2max
HDL2 Placebo 40.8 (3.35) 41.1 (3.30) 1.83 (2.86) 0.701 0.003Tocopherol 43.3 (1.90) 37.4 (1.68) �12.41 (3.89) 0.008
HDL3 Placebo 42.9 (2.54) 42.6 (2.75) �1.02 (1.78) 0.916 0.000Tocopherol 43.9 (2.63) 39.7 (1.82) �8.91 (1.34) 0.001
a-Tocopherol was assessed by HPLC and time1/2max was assessed on a spectropho-tometer.a-Tocopherol is expressed as mmol/L in serum and as nmol/mg protein: Time1/2max isexpressed as min.Results are mean (SEM): n ¼ 15 per group.
a Pre vs. post intervention.b Difference between groups.
Table 2Ex vivo: PON-1 and LCAT activities in serum and HDL2&3.
Group Baseline Week-6 % Change pa pb
PON-1Serum Placebo 26,448 (3842) 25,297 (3039) �0.75 (12.03) 0.600 0.756
Tocopherol 26,707 (1782) 24,316 (1625) �5.94 (10.14) 0.691HDL2 Placebo 1.14 (0.14) 1.12 (0.11) �2.82 (8.14) 0.807 0.027
Tocopherol 1.19 (0.11) 1.00 (0.11) �15.99 (4.25) 0.008HDL3 Placebo 1.29 (0.26) 1.26 (0.24) �0.49 (3.28) 0.441 0.041
Tocopherol 1.28 (0.19) 1.18 (0.25) �13.21 (6.91) 0.035LCATSerum Placebo 0.995 (0.03) 0.950 (0.02) �4.46 (3.18) 0.180 0.309
Tocopherol 0.937 (0.01) 0.922 (0.01) �0.96 (2.52) 0.648HDL2 Placebo 3.58 (0.45) 3.48 (0.26) �2.45 (4.86) 0.776 0.089
Tocopherol 3.74 (0.27) 3.32 (0.33) �11.00 (5.20) 0.050HDL3 Placebo 0.31 (0.07) 0.32 (0.08) 3.82 (3.15) 0.430 0.492
Tocopherol 0.40 (0.10) 0.35 (0.08) �5.13 (8.35) 0.248
PON-1 and LCAT activities were assessed by spectro and fluorimetric assays,respectively.PON-1 arylesterase activity is expressed as U/L in serum and as mU/mg protein inHDL2&3.LCAT activity is expressed as ratio in serum and as ratio/mg protein in HDL2&3.Results are mean (SEM): n ¼ 15 per group.
a Pre vs. post intervention.b Difference between groups.
L. Wade et al. / Atherosclerosis 226 (2013) 392e397 395
3.4. a-Tocopherol concentration (Table 1)
Baseline serum and HDL2&3 a-tocopherol levels were similarbetween the placebo and a-tocopherol groups (p ¼ 0.695, 0.645and 0.608, respectively). Additionally, a-tocopherol was unaffectedfollowing 6-weeks placebo intervention (p > 0.05, for serum andHDL2&3). However, a-tocopherol significantly increased in serumand HDL2&3 following the a-tocopherol intervention (p < 0.05 forserum and HDL2&3). Additionally, this increase was maintained inthe between-group comparisons (p < 0.05, for serum and HDL2&3).
3.5. Oxidation potential (Table 1)
At baseline, the oxidation potential (time1/2max) of HDL2&3 wassimilar between the placebo and a-tocopherol groups (p¼ 0.249 and0.890, respectively). Additionally, following the 6-week interventionperiod, time1/2max in HDL2&3 was unaffected in the placebo group(p > 0.05 for both subfractions). However, time1/2max decreased inboth HDL2&3 in subjects randomised to the a-tocopherol interven-tion (p < 0.05 for both subfractions), thus indicating that a-tocoph-erol was functioning as a pro-oxidant. Furthermore, this enhancedperoxidationwithin the a-tocopherol group remained evident in thebetween-group comparisons (p < 0.05 for both subfractions).
Due to the a-tocopherol group displaying pro-oxidant proper-ties during the oxidation of HDL2&3, the following analyses wereperformed to examine if an increase in a-tocopherol influencedother aspects of HDL function.
3.6. a-Tocopherol and functional aspects of HDL2&3
Apo AI e No differences were identified at baseline or in thewithin and between-group analyses for apo AI concentration inHDL2&3 (p > 0.05 for all comparisons), results not shown.
SAAe No differences were identified at baseline or in the withinor between-group analyses for serum and HDL2&3 (p > 0.05 for allcomparisons), results not shown.
PON-1 (Table 2) e Baseline comparisons of serum and HDL2&3-PON-1were similar between the groups (p¼ 0.945, 0.565 and 0.586,respectively). Additionally, PON-1 activity in serum and HDL2&3 wasunaffected in the placebo group (p> 0.05 for all analyses). However,the activity of PON-1 decreased in HDL2&3 following the a-tocoph-erol intervention (p< 0.05 for both), with this difference remainingevident between groups (p < 0.05 for both subfractions).
CETP e Results identified that CETP activity was similar atbaseline and was unchanged following placebo and a-tocopherolintervention in serum and HDL2&3 (p > 0.05 for all comparisons),results not shown.
LCAT (Table 2) e At baseline, the activity of LCAT was similar inserum and HDL2&3 between the groups (p ¼ 0.140, 0.447 and 0.764,
L. Wade et al. / Atherosclerosis 226 (2013) 392e397396
respectively). Additionally, the activity of LCAT was unaffected inserum and HDL2&3 following the placebo intervention, and in serumand HDL3 following the a-tocopherol intervention (p > 0.05 for allcomparisons). However, LCAT’s activity decreased in HDL2 followingthe a-tocopherol intervention (p < 0.05), which approachedsignificance in the between-group analyses (p ¼ 0.089).
4. Discussion
In vitro investigations e Previous studies have investigated theantioxidant potential of a-tocopherol during the oxidation of themajor lipoproteins VLDL, LDL and HDL. However, research intothe antioxidant potential of a-tocopherol during the oxidation ofHDL’s two main subfractions, HDL2&3, is lacking. It was contendedthat examining the individual HDL subfractions may be moreinformative than investigating total-HDL, particularly as thesesubfractions have differing mechanisms in the prevention of CVD[22,23]. Furthermore, previous studies investigating the antioxi-dant potential of a-tocopherol added this antioxidant to the lipo-proteins following their isolation from serum, and in this scenariothe a-tocopherol was protective towards lipoprotein oxidation[24,25]. However, similarly to our previous suggestions for total-HDL [5], we propose that this mode of in vitro examination maybe erroneous, and would not reflect the in vivo situation. Therefore,as well as repeating the post-incubated experiments, this currentstudy examined the oxidation potential of HDL2&3 when isolatedfrom serum pre-incubated with a-tocopherol, which we suggestmay better reflect the in vivo/ex vivo situation.
Firstly, when a-tocopherol was added to HDL2&3 post-isolationwefound that it protected both subfractions againstoxidation,whichwasin agreement with previous studies on total-HDL [5,26]. Secondly,with regards to the role of a-tocopherol in the pre-incubated scenario,this study found that the incorporation of a-tocopherol into HDL2&3was inversely associated with their susceptibility to oxidation (HDL2,r ¼ �0.843, p < 0.001; HDL3, r ¼ �0.841, p < 0.001), indicating thata-tocopherol was exhibiting an overt pro-oxidant effect. This findingwas in accordancewith the results of our previous in vitro studies [5]and those of Raveh et al. [14] for total-HDL.
We propose that the differing actions of a-tocopherol may berationalised as follows:Normally, HDL is responsible for the removalof lipid peroxides fromsites of oxidation,while PON-1 is responsiblefor their inactivation within HDL [16]. However, when the concen-tration of a-tocopherol is increased within HDL2&3, as in ourpre-incubated scenario, the activity of PON-1 may be reduced,similarly to that presented in our ex vivo study. Thus any preformedlipid peroxides within HDL2&3 would not be inactivated and, viatocopherol mediated peroxidation [27], they would seed the per-oxidation of HDL2&3. Additionally, the positioning of the a-tocoph-erolmayalso be important. In the pre-incubated scenario, the addeda-tocopherol may be fully incorporated within the HDL particlesand thus may be exposed to core preformed lipid peroxides. Whilein the post-incubated scenario, the a-tocopherol may not be fullyincorporated, which may mean that it would not be exposed tothese core preformed lipid peroxides. However, it may be betterplaced to terminate any aqueous derived free radicals as they enterthe HDL particles, therefore helping prevent lipid peroxidation.However, the peroxidation of HDL is of particular importance,especially as evenmildly oxidised HDL has a diminished capacity toparticipate in cholesterol efflux [28] and also loses some of its otherantiatherogenic functions [29].
Ex vivo investigation e This study identified that a-tocopherolsignificantly increased in serum and within HDL2&3 in thea-tocopherol-supplemented group, while it remained unchanged inthe placebo group, thus confirming subject compliance.With regardsto the oxidationpotential ofHDL2&3, this study found that similarly to
the findings from the pre-incubated in vitro study, a 6-weeksupplement of 400 IU/day of a-tocopherol was detrimental to theoxidation potential of both HDL2&3. In accordance with the currentresults, our previous study [5] found that both a- and g-tocopherolincreased the ex vivo oxidation of total-HDL, although this was onlysignificant in theg-tocopherol group (p¼ 0.032).We suggest that thereduced effect in our former a-tocopherol study may be due to itsshorter duration of 4-weeks, as opposed to the 6-weeks of thiscurrent study. This suggests that a 6-week intervention may berequired to fully identifya-tocopherol’s pro-oxidanteffects, althoughmore prolonged intervention periods may be required to investigatethis property fully.
We must also take into account the concentration of a-tocoph-erol utilised in this current study (400 IU/day). This concentrationhas been widely used in studies investigating the cardioprotectiveproperties of a-tocopherol, and is also the concentration commonlytaken by the general public. However, a-tocopherol at or above150 IU/day has been linked to all cause mortalities [30]. Thissuggests, together with the findings presented here, that lowerdoses of a-tocopherol should be investigated more fully, to assess ifin this case that a-tocopherol would be cardioprotective.
Overall and in conclusion, the results presented here may helpexplain the often contradictory and inconclusive findings from thenumerous clinical and randomised control trials with a-tocopherol[9e13], especially as higher concentrations of a-tocopherol may beproducing HDL with reduced cardioprotective properties.
One suchmodificationknown to reduce theprotectivepropertiesof HDL is its association with the inflammatory molecule, SAA [15].This causes HDL to undergo dramatic alterations in terms of struc-ture and composition [15, 17]. However, this study was unable toidentify any changes in serum or HDL2&3-associated SAA followinga-tocopherol intervention. This may be reflective of the differingoxidative properties of this antioxidant. For example, any inflam-matory response mediated by the peroxidised HDL may be negatedby the enhanced antioxidant effects that a-tocopherol affords toVLDLandLDL [5,25]. In this situation, thenet effectwouldbe that theoverall inflammatory response would appear unchanged, althoughthis proposed mechanismwould require further investigation.
By contrast, the current study did identify changes in the activityof PON-1 in both HDL2&3, following a-tocopherol supplementation.As described, the principle role of this enzyme is to prevent theoxidativemodification of apo B containing lipoproteins, particularlyLDL [16]. Therefore, a loss in PON-1’s activity would be indicative ofa proatherogenic change, which may promote CVD. This findingalso supports the increased oxidation potential of HDL2&3 duringtheir copper mediated oxidation. Therefore, in view of these find-ings and similarly to our suggestion for the in vitro pre-incubatedwork, we propose that the increased tocopheryl radicals and/orthe peroxidised lipid within the HDL subfractions may havereduced the activity of PON-1. This would render HDL less able toinactivate oxidised products removed from apo B containing lipo-proteins, which in turn would accelerate the oxidation of HDL.
Another enzyme which is essential for the normal functioning ofHDL is CETP, although an increase in its activity is associated withdetrimental remodelling changes to HDL [3]. However, the currentinvestigation was unable to detect any changes in CETP activityfollowing intervention. Therefore, as CETP does not possess any anti-oxidant activity, these results suggest that the increased tocopherylradicals and/or the peroxidised lipids found within HDL2&3, due toincreased a-tocopherol, did not affect the functioning of this enzyme.
The final HDL-associated enzyme assessed in this current studywas LCAT, whose activity decreased in HDL2 following thea-tocopherol supplementation, a finding which is indicative of anatherogenic change to this HDL subfraction. Therefore, since LCATdoes possess antioxidant properties [3], it can be suggested that
L. Wade et al. / Atherosclerosis 226 (2013) 392e397 397
similarly to a-tocopherol’s role in reducing the activity of PON-1,a-tocopherol may have inactivated this enzyme. This would thenincrease the preformed lipid peroxides within HDL2, which wouldaccelerate the oxidation process.
In conclusion, conflicting results arose from the in vitro investiga-tions carriedoutduring this study, such that increasing concentrationsof a-tocopherol exerted either an antioxidant (when incubated withisolated HDL2&3) or pro-oxidant (when pre-incubated with serumprior to HDL2&3 isolation) effect on HDL2&3. To confirmwhich of theseresults wasmore reflective of the in vivo situation, a 6-week placebo-controlled supplementation studywith a-tocopherol was performed.Identifying that a-tocopherol functioned as a pro-oxidant during theoxidation of HDL2&3. This pro-oxidant effect was further confirmedwhenother functional aspects ofHDL2&3were examined,whereHDL’smajorantioxidantenzymes,namelyPON-1, and toa lesserextentLCAT,were altered to a less cardioprotective phenotype.
The results of this study support a model whereby a-tocopheroltaken as a supplement of 400 IU/day has detrimental pro-oxidanttendencies towards HDL2&3. This would suggest that increasedintake of a-tocopherol may be producing HDL with reduced car-dioprotective properties. This may contribute to the pathophysiologyof CVD, or at the very least may negate any antiatherogenic potentialthat thisantioxidantaffords to theapoBcontaining lipoproteins. Theseresults may help explainwhy intervention studies with a-tocopherolhave been unsuccessful in identifying any cardioprotective effects.
Conflict of interest
No financial support to declare. All authors declare no conflict ofinterest.
References
[1] Bhopal RS, Rafnsson SB, Agyemang C, et al. Mortality from circulatory diseasesby specific country of birth across six European countries: test of concept. EurJ Public Health 2012;22:353e9.
[2] Poli A, Corsini A. Reversible and non-reversible cardiovascular risk in patientstreated with lipid-lowering therapy: analysis of SEAS and JUPITER trials. Eur JIntern Med 2010;21:372e3.
[3] Kontush A, Chapman MJ. Functionally defective high-density lipoprotein:a new therapeutic target at the crossroads of dyslipidemia, inflammation, andatherosclerosis. Pharmacol Rev 2006;58:342e74.
[4] Nunez-Cordoba JM, Martinez-Gonzalez MA. Antioxidant vitamins andcardiovascular disease. Curr Top Med Chem 2011;11:1861e9.
[5] Nadeem N, Woodside JV, Kelly S, Allister R, Young IS, McEneny J. The two facesof alpha- and gamma-tocopherols: an in vitro and ex vivo investigation intoVLDL, LDL and HDL oxidation. J Nutr Biochem 2012;23:845e53.
[6] Nojiri S, Daida H, Inaba Y. Antioxidants and cardiovascular disease: still a topicof interest. Environ Health Prev Med 2004;9:200e13.
[7] Arrol S, Mackness MI, Durrington PN. Vitamin E supplementation increasesthe resistance of both LDL and HDL to oxidation and increases cholesterylester transfer activity. Atherosclerosis 2000;150:129e34.
[8] Halliwell B, Rafter J, Jenner A. Health promotion by flavonoids, tocopherols,tocotrienols, and other phenols: direct or indirect effects? Antioxidant or not?Am J Clin Nutr 2005;81:268Se76S.
[9] Azzi A, Ricciarelli R, Zingg JM. Non-antioxidant molecular functions of alpha-tocopherol (vitamin E). FEBS Lett 2002;22:8e10.
[10] Salonen JT, Nyyssonen K, Salonen R, et al. Antioxidant Supplementation inAtherosclerosis Prevention (ASAP) study: a randomized trial of the effect ofvitamins E and C on 3-year progression of carotid atherosclerosis. J Intern Med2000;248:377e86.
[11] Boaz M, Smetana S, Weinstein T, et al. Secondary prevention with antioxi-dants of cardiovascular disease in endstage renal disease (SPACE): rando-mised placebo-controlled trial. Lancet 2000;356:1213e8.
[12] Hodis NH, Mackm WJ, LaBree L, et al. Alpha-tocopherol supplementation inhealthy individuals reduces low-density lipoprotein oxidation but notatherosclerosis: the Vitamin E Atherosclerosis Prevention Study (VEAPS).Circulation 2002;106:1453e9.
[13] Lonn E, Bosch J, Yusuf S, et al. Effects of long-term vitamin E supplementationon cardiovascular events ad cancer: a randomized controlled trial. J Am MedAssoc 2005;293:1338e47.
[14] Raveh O, Pinchuk I, Schnitzer E, Fainaru M, Schaffer Z, Lichtenberg D. Kineticanalysis of copper-induced peroxidation of HDL, auto accelerated andtocopherol-mediated peroxidation. Free Radic Biol Med 2000;29:131e46.
[15] Artl A, Marsche G, Lestavel S, Sattler W,Malle E. Role of serum amyloid A duringmetabolism of acute-phase HDL bymacrophages. Arterioscler Thromb Vasc Biol2000;20:763e72.
[16] Kontush A, de Faria EC, Chantepie S, Chapman MJ. Antioxidative activity ofHDL particle subspecies is impaired in hyperalphalipoproteinemia: relevanceof enzymatic and physicochemical properties. Arterioscler Thromb Vasc Biol2004;24:526e33.
[17] Van Lenten BJ, Hama SY, de Beer FC, et al. Anti-inflammatory HDL becomespro-inflammatory during the acute phase response. Loss of protective effect ofHDL against LDL oxidation in aortic wall cell cocultures. J Clin Invest 1995;96:2758e67.
[18] Brites FD, Bonavita CD, De Geitere C, et al. Alterations in the main steps ofreverse cholesterol transport in male patients with primary hyper-triglyceridemia and low HDL-cholesterol levels. Atherosclerosis 2000;152:181e92.
[19] McPherson PA, Young IS, McKibben B, McEneny J. High density lipoproteinsubfractions: isolation, composition, and their duplicitous role in oxidation.J Lipid Res 2007;48:86e95.
[20] Craft NE. Carotenoid reversed-phase high-performance liquid chroma-tography methods: reference compendium. Meth Enzymol 1992;213:185e205.
[21] Hasselwander O, McEneny J, McMaster D, et al. HDL composition and HDLantioxidant capacity in patients on regular haemodialysis. Atherosclerosis1999;143:125e33.
[22] Movva R, Rader DJ. Laboratory assessment of HDL heterogeneity and function.Clin Chem 2008;54:788e800.
[23] Kontush A, Chapman MJ. Antiatherogenic function of HDL particle subpopu-lations: focus on antioxidative activities. Curr Opin Lipidol 2010;21:312e8.
[24] Mackness MI, Durrington PN. HDL, its enzymes and its potential to influencelipid peroxidation. Atherosclerosis 1995;115:243e2531995.
[25] Arai H, Uchida K, Fukunaga K, et al. Effect of alpha-tocopherol on the oxidativemodification of apolipoprotein E in human very-low-density lipoprotein.Biosci Biotechnol Biochem 2003;67:402e5.
[26] Sakai M, Miyazaki A, Sakamoto Y, Shichiri M, Horiuchi S. Cross-linking ofapolipoproteins is involved in a loss of the ligand activity of high densitylipoprotein upon Cu(2þ)-mediated oxidation. FEBS Lett 1992;314:199e202.
[27] Thomas SR, Stocker R. Molecular action of vitamin E in lipoprotein oxidation:implications for atherosclerosis. Free Radic Biol Med 2000;28:1795e805.
[28] Nagano Y, Arai H, Kita T. High density lipoprotein loses its effect to stimulateefflux of cholesterol from foam cells after oxidative modification. Proc NatlAcad Sci U S A 1991;88:6457e61.
[29] McCall MR, Tang JY, Bielicki JK, Forte TM. Inhibition of lecithin-cholesterolacyltransferase and modification of HDL apolipoproteins by aldehydes. Arte-rioscler Thromb Vasc Biol 1995;15:1599e606.
[30] Miller ER, Pastor-Barriuso R, Dalal D, Riemersama RA, Appel LJ, Guallar E.Meta-analysis: high-dosage vitamin E supplementation may increase all-casemortality. Ann Intern Med 2005;142:37e46.