The antiandrogenic activity of pyrethroid pesticides cyfluthrin and β-cyfluthrin
Transcript of The antiandrogenic activity of pyrethroid pesticides cyfluthrin and β-cyfluthrin
Reproductive Toxicology 25 (2008) 491–496
Contents lists available at ScienceDirect
Reproductive Toxicology
journa l homepage: www.e lsev ier .com/ locate / reprotox
The antiandrogenic activity of pyrethroid pesticides cyfluthrin and �-cyfluthrin
Jun Zhang, Wei Zhu, Yifan Zheng, Jun Yang, Xinqiang Zhu ∗
Department of Toxicology, School of Medicine, Zhejiang University, 388 Yuhangtang Road, Hangzhou 310058, Zhejiang Province, PR China
ivo anthrinterme He
ivo. Mtructuow tA-kb
se oferal p/kg d
incre< cyflnic ch.
a r t i c l e i n f o
Article history:Received 28 January 2008Received in revised form 8 April 2008Accepted 6 May 2008Available online 15 May 2008
Keywords:PyrethroidAntiandrogenAndrogen receptorHershberger assayTranscriptional activation assay
a b s t r a c t
Herein we describe in vof two pyrethroids, cyfluMDA-kb2, was used to decyfluthrin in vitro, and ththe two pyrethroids in vand �-cyfluthrin to four sbifenthrin. Our results shinduced AR activity in MDand �-cyfluthrin, at a doventral prostate, dorsolatCyfluthrin at dose of 12 mgaccessory sex tissues. Thecyfluthrin < cypermethrinare moderate antiandrogepartly by antagonizing AR
1. Introduction
Endocrine Disrupting Chemicals (EDCs) are of major concern totoxicologists and environmental researchers because of their harm-ful effects to human and wildlife reproductive system. As a subclassof the EDCs, environmental antiandrogens can cause abnormallydeveloped reproductive organs and reproductive dysfunction suchas hypospadias, cryptorchidism, decreased semen quality, alter-ations in sex differentiation, and testis cancer [1–7]. Previous dataindicated that antiandrogens exert their activity mainly throughthe interaction with the androgen receptors (AR) in target cells[8,9]. Several pesticides have been reported to possess antian-drogenic activity, including p,p′-DDE, vinclozolin, procymidone,linuron, methoxychlor, fenitrothion, prochloraz, and fenarimol, asshown in in vivo [10–15] and in vitro assays [5,7,12,14–16]. Thesestudies of antiandrogenic activity of commonly used chemicals arecritically important to protect human and ecological health.
Synthetic pyrethroids are a group of insecticides similar instructure to the pyrethrins. Because of their high toxicity to insectsand low potency in mammals, pyrethroids are widely used through
∗ Corresponding author. Tel.: +86 571 88208143; fax: +86 571 88208143.E-mail address: [email protected] (X. Zhu).
0890-6238/$ – see front matter © 2008 Elsevier Inc. All rights reserved.doi:10.1016/j.reprotox.2008.05.054
d in vitro assays to investigate the suspected antiandrogenic activityand �-cyfluthrin. A stably transfected, androgen-responsive cell line,
ine the androgen receptor (AR) antagonistic effects of cyfluthrin and �-rshberger assay was utilized to detect the antiandrogenic potential oforeover, we also compared the antiandrogenic activities of cyfluthrinrally related pyrethoids: permethrin, cypermethrin, �-cypermethrin and
hat cyfluthrin and �-cyfluthrin can block 5-dihydrotestosterone (DHT)-2 cells. In the Hershberger assay, cyfluthrin, at doses of 18 and 54 mg/kg,36 mg/kg, caused significant decrease in the weight of seminal vesicle,rostate, LABC, Cowper’s glands, though not significant in glans penis. �-ecreased only the weight of seminal vesicle and had no effect on the other
ase rank of antiandrogenic activity was: �-cypermethrin < permethrin < �-uthrin < bifenthrin < flutamide. In conclusion, cyfluthrin and �-cyfluthrinemicals in our experiments, and they elicit antiandrogenic effects at least
© 2008 Elsevier Inc. All rights reserved.
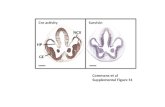
out the world to control insects in agriculture and in room environ-ments. Cyfluthrin [�-cyano(4-fluoro-3-phenoxyphenyl)methyl3-(2,2-dichloroethenyl)-2,2-dimethylcyclopropanecarboxylate]and �-cyfluthrin (optical isomer of cyfluthrin, including four
diastereoisomeric pairs of enantiomers) are two syntheticpyrethroids (Fig. 1). These two chemicals share a similar CASnumber, 68359–37–5. Up to the present, it is still not clear if thesetwo pyrethroids have any antiandrogenic activity. So, in the cur-rent study, we examined antiandrogenic effects of cyfluthrin and�-cyfluthrin, using reporter gene assays in vitro and Hershbergerassays in vivo.2. Materials and methods
2.1. Test compounds
Testosterone propionate (TP) (CAS no. 57–85–2) was purchased from UniKem,Denmark, flutamide (CAS no. 13311–84–7) and hydrotestosterone (DHT) (CASno. 521–18–6) were purchased from Fluka, Switzerland. Hydroxyflutamide (OHF,purity < 99%) was provided by Fudan University, China. Cyfluthrin (purity = 92.6%),�-cyfluthrin (purity = 97%), permethrin, cypermethrin, �-cypermethrin and bifen-thrin were gifts from Huifeng, China. The purities of above chemicals were providedby the supplier. Peanut oil was purchased from Luhua Group, China.
2.2. Cell model
MDA-kb2 cell line was provided by ATCC, and it has been stably transfectedwith the pMMTV.neo.luc reporter gene plasmid by Professor Wilson in USEPA
492 J. Zhang et al. / Reproductive Toxicology 25 (2008) 491–496
) cyflu
Fig. 1. The structures of (A[17]. The cells were maintained at 37 ◦C without CO2 in L-15 media supple-mented with 10% FBS, 100 U/ml penicillin, 100 �g/ml streptomycin, and 0.25 �g/mlamphotericin B.
2.3. Luciferase activity assay
When MDA-kb2 cells reached nearly 80% of confluence, the cells weredosed with various concentrations of test compounds with or without DHT. Testcompounds were prepared in ethanol as 1000 �g/ml stock solutions, and final con-
◦
centration of ethanol in medium never exceeded 0.1%. Cells were incubated at 37 Cwithout CO2 overnight for 24 h. After 24 h incubation, dosing medium was removedfrom each plate, cells were washed once with phosphate-buffered saline (PBS) atroom temperature, and then 25 �l lysis buffer (Promega, Madison, WI, USA) wasadded per well and incubated for 25 min. When cells were lysed, 100 �l luciferasereaction agents (Promega, Madison, WI, USA) were added and luciferase activitywas determined using Wallac Victor2 1420 Multilabel Counter (PerkinElmer LifeSciences, Turku, Finland) following the manufacturer’s instructions.2.4. Animals
Three-week-old Sprague-Dawley male rats were purchased from the Experi-mental Animal Center, Zhejiang Academy of Medical Sciences, Hangzhou, China.They were fed on standard laboratory diet, with water ad libitum, and were keptin an animal room with 12 h light/dark cycle, controlled temperature 22 ± 1 ◦C andrelative humidity of 55 ± 5%. After 7 days adaptive period, rats were castrated andallowed to recover for 2 weeks.
2.5. Hershberger assay
After 2 weeks recovery period, rats were weighed and randomly assigned toexperiment 1 (9 groups, n = 6/group) and experiment 2 (8 groups, n = 6/group).In experiment 1, group 1 was the sham-operated group; group 2 served as thevehicle control group, given only 5 ml/kg body weight (BW) peanut oil orally;groups 3–5 received oral doses of 6, 18, or 54 mg/kg cyfluthrin per day, respec-tively; groups 6–8 received oral doses of 4, 12, or 36 mg/kg �-cyfluthrin per day,
thrin and (B) �-cyfluthrin.
respectively. Group 9 was the positive control group, and it received oral dose offlutamide (50 mg/kg per day). Groups 2–9 received TP subcutaneously (0.5 mg/kgper day) before test chemicals were administered. In experiment 2, the eight groupswere TP (0.5 mg/kg s.c.) + peanut oil (control group, 5 ml/kg, orally), TP + flutamide(50 mg/kg orally), TP + cyfluthrin (50 mg/kg orally), TP + �-cyfluthrin (50 mg/kgorally), TP + cypermethrin (50 mg/kg orally), TP + �-cypermethrin (50 mg/kg orally),TP + permethrin (50 mg/kg orally), TP + bifenthrin (13.5 mg/kg orally), respectively.The test chemicals and TP were suspended in peanut oil. The daily amount ofadministration was 5 ml/kg BW for oral gavage and 0.5 ml/kg BW for subcutaneous
injections of TP. The doses for the test chemicals were determined from literatureinformation and pilot study (data not shown). All groups were dosed from 6 weeksold, and the dosing period was 7 days. Rats were weighed before dosing every day.After the last dose was administered, rats were anaesthetized using 50 mg/kg pen-tobarbital sodium followed by exsanguinations. Blood from each rat was collectedin 5 ml tubes, and the serum was obtained by centrifugation at 14,000 × g for 10 minand stored at −80 ◦C until hormone analyses. The seminal vesicles, ventral prostate,dorsolateral prostate, levator ani/bulbocavernosus (LABC) muscle, Cowper’s glands,glans penis, liver, paired adrenal glands, spleen and paired kidneys were dissectedand weighed.2.6. Hormone analysis
Rat serum testosterone was analyzed by time-resolved fluorescence using com-mercially available FIA kits (PerkinElmer Life Sciences, Turku, Finland). The serumwas prepared as previously described [18]. All samples were measured in duplicatein the same assay.
2.7. Statistical analysis
Data for initial body weights, final body weights, serum testosterone levelsand gene expression were analyzed using ANOVA test when variance of the datawere proved to be homogeneous, and, if not, using nonparametric analysis of vari-ance. Organ weights were analyzed using ANCOVA with the final body weight as acovariate.
e Toxi
J. Zhang et al. / ReproductivFig. 2. Androgen receptor inhibition activity of cyfluthrin (CYF), �-cyfluthrin (B-CYF) and hydroxyflutamide (OHF) in the AR reporter gene assay with 1 nM DHT. Datarepresent the mean ± S.E.M. of four independent experiments. *P < 0.05 comparedwith the value of 1 nM DHT, **P < 0.01 compared with the value of 1 nM DHT.
3. Results
3.1. In vitro
DHT increased AR activity and induced luciferase reportergene expression in a concentration-dependent manner (data notshown), but neither cyfluthrin nor �-cyfluthrin increased AR activ-ity in the absence of DHT (data not shown). As shown in Fig. 2,cyfluthrin and �-cyfluthrin blocked DHT-induced transcriptionalactivation in a dose-dependent manner at concentrations from1.0 × 10−7 to 1.0 × 10−5 mol/L (P < 0.05). The in vitro potencies ofcyfluthrin and �-cyfluthrin as AR antagonists were less than thatof the antiandrogen hydroxyflutamide.
In addition to cyfluthrin and �-cyfluthrin, we also detectedthe in vitro antiandrogenic activity of other four pyrethroidscypermethrin, �-cypermethrin, permethrin and bifenthrin at 1.0 ×10−5 mol/L. Our results showed that cypermethrin, permethrin andbifenthrin significantly inhibited DHT-induced transcriptional acti-vation, but that �-cypermethrin had no effect on it (Fig. 3).
3.2. In vivo
3.2.1. General observations and body weightsWe found no mortality or treatment-related clinical signs in any
of the groups. There were no significant difference between the
Fig. 3. Androgen receptor inhibition activity of six pyrethroids, cyfluthrin (CYF), �-cyfluthrin (B-CYF), cypermethrin (CYP), �-cypermethrin (B-CYP), permethrin (PER)bifenthrin (BIF), and hydroxyflutamide (OHF) in the AR reporter gene assay with1 nM DHT. Data represent the mean ± S.E.M. of four independent experiments.*P < 0.05 compared with the value of 1 nM DHT.
cology 25 (2008) 491–496 493
Table 1Effect of treatments on rat body weights in Hershberger assay (x̄ ± s, g)
Treatments Initial body weight Final body weight Weight gain
Sham-operated 219.5 ± 11.3 284.5 ± 12.6 64.8 ± 9.4TP + peanut oil 215.1 ± 11.0 287.4 ± 11.5 72.3 ± 8.6
TP + cyfluthrin6 mg/kg 224.5 ± 16.2 292.8 ± 15.8 68.3 ± 9.518 mg/kg 222.3 ± 20.4 291.5 ± 19.4 69.2 ± 13.854 mg/kg 215.1 ± 18.0 283.8 ± 12.8 68.7 ± 16.4
TP + �-cyfluthrin4 mg/kg 215.4 ± 9.6 277.7 ± 7.4 62.3 ± 8.112 mg/kg 224.7 ± 16.4 296.1 ± 20.3 71.4 ± 11.736 mg/kg 226.2 ± 12.2 288.2 ± 14.7 62.0 ± 7.0
TP + flutamide 213.6 ± 14.4 279.1 ± 10.8 65.5 ± 6.8
initial body weights, the final body weights and weight gains ofthe groups compared with the peanuts oil control group (P > 0.05)(Table 1).
3.2.2. General organ weightsCompared with the TP plus peanut oil control group, weights
of liver, kidney and spleen in flutamide and cyfluthrin groups haveno significant difference (P > 0.05). Pairs of adrenal glands weightsof the 36 mg/kg−1 per day �-cyfluthrin plus TP group were sig-nificantly higher than those of peanut oil plus TP control group(P < 0.05), (Table 2).
3.2.3. Sex accessory tissue weights and hormone levelsAs expected, TP increased the accessory sex organ weights to
normal levels compared to hormone deficient peanut oil controlgroup (data not shown). Flutamide, a known antiandrogen, inhib-ited the TP-induced growth of accessory sex organs significantly,as seen in seminal vesicle, ventral prostate, dorsolateral prostate,LABC, Cowper’s glands, and glans penis (P < 0.05). As with flutamide,cyfluthrin at the dose of 18 and 54 mg/kg and �-cyfluthrin at adose of 36 mg/kg caused statistically significant decreases (com-pared with TP plus peanut oil control group) in the weights ofseminal vesicle, ventral prostate, dorsolateral prostate, LABC, Cow-per’s glands (P < 0.05), though for glans penis the difference was notsignificant (P > 0.05). �-Cyfluthrin at a dose of 12 mg/kg decreased(P < 0.05) the weight only of seminal vesicle and had no effect onthe other accessory sex tissues (P > 0.05) (Table 3). No changes wereobserved in the testosterone levels of the groups (data not shown).
We also measured the antiandrogenic activity of cyperme-thrin, �-cypermethrin, permethrin and bifenthrin in vivo. Similarto in vitro assays, �-cypermethrin did not reduce the weightof main androgen-dependent tissues remarkably (P > 0.05), while
Table 2Effect of treatments on rat organ weights in Hershberger assay (x̄ ± s)
Treatments Liver (g) Kidney (g) Adrenals (mg) Spleen (g)
Sham-operated 10.62 ± 0.51 2.06 ± 0.14 55.5 ± 7.3 0.650 ± 0.104TP + peanut oil 10.80 ± 1.17 2.15 ± 0.23 48.3 ± 5.1 0.657 ± 0.153
TP + cyfluthrin6 mg/kg 11.39 ± 1.04 2.25 ± 0.16 52.8 ± 14.4 0.809 ± 0.25418 mg/kg 12.33 ± 1.34 2.45 ± 0.27 57.9 ± 14.3 0.755 ± 0.11654 mg/kg 11.37 ± 0.93 2.19 ± 0.24 54.3 ± 11.9 0.699 ± 0.094
TP + �-cyfluthrin4 mg/kg 12.35 ± 1.36 2.24 ± 0.18 52.2 ± 9.2 0.650 ± 0.04412 mg/kg 10.64 ± 0.27 2.12 ± 0.32 57.5 ± 15.5 0.684 ± 0.15336 mg/kg 11.99 ± 0.87 2.36 ± 0.30 65.3 ± 7.8* 0.677 ± 0.165
TP + flutamide 11.48 ± 0.78 2.12 ± 0.26 57.6 ± 12.6 0.618 ± 0.176
* Significantly different from the control group (peanut oil; P < 0.05).
e Toxi
ershbe
latera
± 14± 23
± 9.2± 14± 8.8
± 10± 21± 9.3
± 5.7
494 J. Zhang et al. / Reproductiv
Table 3Effect of treatments on rat androgen relevant sex accessory glands and tissues in H
Treatments Seminal vesicle Ventral prostate Dorso
Sham-operated 207.3 ± 38.9 139.2 ± 25.7 131.2TP + peanut oil 205.6 ± 48.6 141.4 ± 22.8 131.0
TP + cyfluthrin6 mg/kg 181.2 ± 7.6 118.4 ± 11.4 108.818 mg/kg 110.4 ± 19.8* 93.6 ± 17.9* 83.854 mg/kg 87.3 ± 6.7* 74.0 ± 5.1* 69.0
TP + �-cyfluthrin4 mg/kg 194.4 ± 5.5 134.2 ± 7.9 121.612 mg/kg 143.4 ± 19.3* 124.4 ± 20.4 110.436 mg/kg 111.2 ± 10.3* 96.6 ± 5.7* 83.2
TP + flutamide 57.2 ± 23.8* 42.4 ± 8.7* 36.4
* Significantly different from peanut oil control group, P < 0.05.
cypermethrin, permethrin and bifenthrin had significant antian-drogenic activity (P < 0.05) (Fig. 4).
4. Discussion
It is clear that AR regulates male embryonic development andsex differentiation by acting as an androgen-inducible transcrip-tion factor. The cytoplasmic AR is activated after interacting withandrogens and then translocated to the nucleus, where it binds toandrogen responsive elements (AREs) in the promoters of targetgenes [19]. Even transient disruption of these processes can result inirreversible developmental defects [7]. Identification of chemicalswith the potential to disrupt normal androgen action is critical toprotect human and ecological health. At present, several in vitro andin vivo screening assays capable of detecting antiandrogen activityof chemical compounds have been well developed [20–22].
In vitro transcriptional activation assays are preferred overreceptor binding assays, because agonist and antagonist activitycan be distinguished. The ability of increasing concentrations ofchemical to reduce agonist-stimulated expression is indicative ofantagonist activity. MDA-kb2 cells are human breast cancer cellsthat are stably transfected with a MMTV-luciferase reporter genethat can be activated by chemicals acting through either AR or glu-cocorticoid receptor (GR). The cells have been developed by USEPAas a Tier-I screening assay of EDCs [17] and used in much previousantiandrogenic research [23–26]. AR- and GR-mediated ligands canbe distinguished by the co-administration of OHF, which is an AR
but not a GR antagonist [17]. In our study, none of the chemicalswe used induced or inhibited the response of MMTV-luciferasereporter gene co-administrated with OHF (data not shown). Wetherefore examined the antiandrogenic activity of cyfluthrin and�-cyfluthrin using MDA-kb2 cells. DHT at 1 nM induced expressionof the reporter gene, illustrating the high sensitivity of this assay.The results showed that cyfluthrin and �-cyfluthrin can inhibitDHT-induced AR activity significantly,The Hershberger assay is a powerful tool for the screening ofEDCs in vivo; it has been recommended by the Endocrine DisruptorScreening and Testing Advisory Committee (EDSTAC) [20] and theOrganization for Economic Co-operation and Development (OECD)[27]. To detect antiandrogen activity of chemicals, the weights ofandrogen-dependent sex accessory tissues (such as seminal vesicle,ventral prostate, dorsolateral prostate, LABC, and Cowper’s glands,etc.) were measured [28–30]. Compared with normal male rats, cas-trated male rats are rid of the classic endocrine feedback pathwaysand thus show more sensitivity and higher specificity to AR-mediated changes in androgen-dependent organs [22]. Althoughlow-dose (6 mg/kg) cyfluthrin had no effects on the sex accessorytissues, cyfluthrin at higher doses (18 and 54 mg/kg) remarkably
cology 25 (2008) 491–496
rger assay (mg, x̄ ± s)
l prostate LABC Cowper’s glands Glans penis
.8 312.2 ± 21.1 14.7 ± 4.1 62.9 ± 11.4.1 302.8 ± 33.3 16.0 ± 2.6 68.2 ± 10.1
319.4 ± 35.0 19.0 ± 1.0 75.2 ± 11.9.7* 210.6 ± 12.6* 13.2 ± 1.9* 62.4 ± 16.8* 191.2 ± 23.7* 11.5 ± 1.7* 68.8 ± 20.0
.6 333.6 ± 20.9 17.0 ± 1.0 65.6 ± 7.8.3 332.4 ± 36.6 17.4 ± 2.4 64.4 ± 15.1* 243.2 ± 16.9* 11.4 ± 3.6* 65.0 ± 20.7
* 155.0 ± 46.6* 6.6 ± 2.1* 54.2 ± 8.3*
reduced the weights of seminal vesicle, ventral prostate, dorsolat-eral prostate, LABC, and Cowper’s glands (Table 3). For �-cyfluthrin,only the highest dose (36 mg/kg) showed a significant reduction ofweights of the same sex accessory tissues (Table 3). Similar to theresults of our previous research [31], seminal vesicle was the mostsensitive tissue, as judged from the fact that seminal vesicle weightwas the only endpoint affected by the middle dose (18 mg/kg) of�-cyfluthrin.
Permethrin and bifenthrin belong to Type I pyrethroid pesti-cides, cypermethrin, �-cypermethrin, cyfluthrin and �-cyfluthrinbelong to Type II pyrethroid pesticides. Very few studies havereported that the antiandrogenic activities of pyrethroid pesti-cides, and the results are contradictory. For example, bifenthrin,permethrin, and cypermethrin have been reported to show antian-drogenic activities in in vivo or in vitro assays [26,32–35]. In contrast,Kunimatsu et al. [36] found that permethrin did not have antiandro-genic effects in vivo, and Kim et al. [37] reported that cypermethrincould not inhibit DHT-induced transactivation in three cell lines(LNCaP, PC3/AR+ and 22Rv1). To compare the antiandrogenic activ-ities of cyfluthrin and �-cyfluthrin to four other structurally relatedpyrethoids: permethrin, cypermethrin, �-cypermethrin and bifen-thrin, we used both in vitro transcriptional activation assays and invivo Hershberger assays. In our results, permethrin, cypermethrin,and bifenthrin were shown to increase transcriptional activityvia AR in vitro and remarkably reduce the weights of sex acces-sory tissues in vivo. Considering our results of in vitro and in vivoassays, we could rank the increase in antiandrogenic activity of the
chemicals we tested as follows: �-cypermethrin < permethrin < �-cyfluthrin < cypermethrin < cyfluthrin < bifenthrin < flutamide.Permethrin, cypermethrin, and cyfluthrin have similar three-dimensional structure and only differ at one or a few groups.Cypermethrin has a �-cyano group at the benzylic carbon on thebase of permethrin, and addition of a p-fluoro substituent on thebenzyl ring of cypermethrin gives cyfluthrin [38]. The insectici-dal activity of cypermethrin and cyfluthrin are more than two orthree folds higher than permethrin. Previous studies have reportedthat permethrin showed antiandrogen effects in vitro [32] and invivo [33]; our previous experiments [34] and Xu et al. [35] havedemonstrated that cypermethrin has antiandrogen effects in vitroand in vivo assays. Currently, we also find that permethrin andcyfluthrin have antiandrogenic effects in vitro and in vivo. Thesefindings suggest that antiandrogenic activity may be inherent inthe core structure of pyrethroid.
Additionally, we have demonstrated that optical isomers havedifferent effects on the antiandrogenic activity of cypermethrinand cyfluthrin. Previous data from our laboratory had shown that�-cypermethrin has no antiandrogenic activity at all [34], while�-cyfluthrin has significant AR antagonistic activity in both in
J. Zhang et al. / Reproductive Toxicology 25 (2008) 491–496 495
weighs with
Fig. 4. The effects of six structurally related pyrethroids on sex accessory tissueand glans penis weights in sham-operated and castrated animals treated for 7 day
(FLU, 50 mg/kg orally), TP + cyfluthrin (CYF, 50 mg/kg orally), TP + �-cyfluthrin (B-CYF, 50 m50 mg/kg orally), TP + permethrin (PER, 50 mg/kg orally), TP + bifenthrin (BIF, 13.5 mg/kgof the castrated CTL group.vitro and in vivo assays. Although these structural changes actu-ally increase the insecticidal activity of these compounds [39], wefind the reverse trend in activity of these optical isomers in term ofrank of antiandrogenic effects.
Conflict of interest
None.
Acknowledgments
The authors would like to thank Professor V.S. Wilson (USEPA)for his help in processing our in vitro MDA-kb2 cell experiments,and the Huifeng Company (China) for providing all the pyrethroidswe used in the experiment. This work was supported in part bygrants from the National Natural Science Foundation of China (no.30471472) and Natural Science Foundation of Zhejiang Province
ts. Seminal vesicle, ventral prostate, dorsolateral prostate, LABC, Cowper’s glandTP (0.5 mg/kg s.c.) + peanut oil (control group, CTL, 5 ml/kg, orally), TP + flutamide
g/kg orally), TP + cypermethrin (CYP, 50 mg/kg orally), TP + �-cypermethrin (B-CYP,orally). Data represent the mean ± S.E.M. (n = 6). *P < 0.05 compared with the value
(no. M303870) to Xinqiang Zhu. The authors are grateful to Dr. R.Wohlhueter, recently retired from the Centers for Disease Controland Prevention (CDC, Atlanta, GA), for his editorial assistance.
References
[1] Weidner IS, Møller H, Jensen TK, Skakkebæk NE. Cryptorchidism and hypospa-dias in sons of gardeners and farmers. Environ Health Perspect 1998;106:793–6.
[2] Garcia-Rodriguez J, Garcia-Martin M, Nogueras-Ocana M, de Dios Luna-del-Castillo J, Espigares Garcia M, Olea N, et al. Exposure to pesticides andcryptorchidism: geographical evidence of a possible association. EnvironHealth Perspect 1996;104:1090–5.
[3] Swan SH, Elkin EP, Fenster L. The question of declining sperm density revis-ited: an analysis of 101 studies published 1934–1996. Environ Health Perspect2000;108:961–6.
[4] Møller H, Friis S, Kjær SK. Survival of Danish cancer patients 1943–1987. Malegenital organs. APMIS Suppl 1993;33:122–36.
[5] Gray Jr LE, Ostby J, Kelce WR. Developmental effects of an environmental antian-drogen: the fungicide vinclozolin alters sex differentiation of the male rat.Toxicol Appl Pharmacol 1994;129:46–52.
e Toxi
[
[
[
[
[
[
[
[
[
[
[
496 J. Zhang et al. / Reproductiv
[6] Kelce WR, Monosson E, Gamcsik MP, Laws SC, Gray Jr LE. Environmental hor-mone disruptors: evidence that vinclozolin developmental toxicity is mediatedby antiandrogenic metabolites. Toxicol Appl Pharmacol 1994;126:276–85.
[7] Kelce WR, Stone CR, Laws SC, Gray Jr LE, Kemppainen JA, Wilson EM. Persis-tent DDT metabolite p,p’-DDE is a potent androgen receptor antagonist. Nature1995;375:581–5.
[8] Sohoni P, Sumpter JP. Several environmental oestrogens are also anti-androgens. J Endocrinol 1998;158:327–39.
[9] Vinggaard AM, Joergensen EC, Larsen JC. Rapid and sensitive reporter geneassays for detection of antiandrogenic and estrogenic effects of environmentalchemicals. Toxicol Appl Pharmacol 1999;155:150–60.
[10] Gray Jr LE, Wolf C, Lambright C, Mann P, Price M, Cooper RL, et al. Administra-tion of potentially antiandrogenic pesticides (procymidone, linuron, iprodione,chlozolinate, p,p′-DDE, and ketoconazole) and toxic substances (dibutyl anddiethylhexyl phthalate, PCB, 169, and ethane dimethane sulphonate) duringsexual differentiation produces diverse profiles of reproductive malformationsin the male rat. Toxicol Ind Health 1999;15:94–118.
[11] Ostby J, Kelce WR, Lambright C, Wolf CJ, Mann P, Gray Jr LE. The fungi-cide procymidone alters sexual differentiation in the male rat by acting asan androgen-receptor antagonist in vivo and in vitro. Toxicol Ind Health1999;15:80–93.
12] Lambright C, Ostby J, Bobseine K, Wilson V, Hotchkiss AK, Mann PC, et al.Cellular and molecular mechanisms of action of linuron: an antiandrogenicherbicide that produces reproductive malformations in male rats. Toxicol Sci2000;56:389–99.
[13] Tamura H, Maness SC, Reischmann K, Dorman DC, Gray Jr LE, Gaido KW. Andro-
gen receptor antagonism by the organophosphate insecticide fenitrothion.Toxicol Sci 2001;60:56–62.[14] Vinggaard AM, Nellemann C, Dalgaard M, Jørgensen EB, Andersen HR. Antian-drogenic effects in vitro and in vivo of the fungicide prochloraz. Toxicol Sci2002;69:344–53.
[15] Vinggaard AM, Jacobsen H, Metzdorff SB, Andersen HR, Nellemann C. Antian-drogenic effects in short-term in vivo studies of the fungicide fenarimol.Toxicology 2005;207:21–34.
[16] Maness SC, McDonnell DP, Gaido KW. Inhibition of androgen receptor-dependent transcriptional activity by DDT isomers and methoxychlor in HepG2human hepatoma cells. Toxicol Appl Pharmacol 1998;151:135–42.
[17] Wilson VS, Bobseine K, Lambright CR, et al. A novel cell line, MDA-kb2,that stably expresses an androgen- and glucocorticoid-responsive reporterfor the detection of hormone receptor agonists and antagonists. Toxicol Sci2002;66(1):69–81.
[18] Birkhoj M, Nellemann C, Jarfelt K, Jacobsen H, Andersen HR, Dalgaard M, et al.The combined antiandrogenic effects of five commonly used pesticides. ToxicolAppl Pharmacol 2004;201:10–20.
[19] Chatterjee B. The role of the androgen receptor in the development of prostatichyperplasia and prostate cancer. Mol Cell Biochem 2003;253:89–101.
20] U.S. Environmental Protection Agency. 1998. Endocrine Disruptor Screen-ing and Testing Advisory Committee (EDSTAC) Final Report. EPA/743/R-98/003. Available:http://www.epa.gov/oscpmont/oscpendo/pubs/edspoverview/finalrpt.htm [accessed 15 January, 2008].
21] Owens W, Zeiger E, Walker M, et al. The OECD program to validate the rat Her-shberger bioassay to screen compounds for in vivo androgen and antiandrogenresponses. Phase 1: use of a potent agonist and a potent antagonist to test thestandardized protocol. Environ Health Perspect 2006;114:1259–65.
[
[
[
[
[
[
[
[
[
[
cology 25 (2008) 491–496
22] Owens W, Gray LE, Zeiger E, et al. The OECD program to validate the ratHershberger bioassay to screen compounds for in vivo androgen and antian-drogen responses: phase 2 dose-response studies. Environ Health Perspect2007;115:671–8.
23] Ma R, Cotton B, Lichtensteiger W, Schlumpf M. UV Filters with antagonisticaction at androgen receptors in the MDA-kb2 cell transcriptional-activationassay. Toxicol Sci 2003;74:43–50.
24] Satoh K, Nonaka R, Ohyama K, Nagai F. Androgenic and antiandrogenic effectsof alkylphenols and parabens assessed using the reporter gene assay with sta-bly transfected CHO-K1 cells (AR-EcoScreen System). J Health Sci 2005;51:557–68.
25] Stoker TE, Cooper RL, Lambright CS, Wilson VS, Furr J, Gray LE. In vivo and invitro anti-androgenic effects of DE-71, a commercial polybrominated diphenylether (PBDE) mixture. Toxicol Appl Pharmacol 2005;207:78–88.
26] Zhu W, Zheng YF, Zhu HJ, Zhu XQ. The antiandrogenic effect and mechanism ofbifenthrin. J Toxicol 2006;20:305–7 (in Chinese).
27] OECD. The Second Meeting of the OECD Validation Management Group (VMG)for the Screening and Testing of Endocrine Disrupters; 2000. Paris: Organisationfor Economic Co-operation and Development.
28] Hershberger L, Shipley E, Meyer R. Myotrophic activity of 19-nortestosteroneand other steroids determined by modified levator ani muscle method. ProcSoc Exp Biol Med 1953;83:175–80.
29] Gray Jr LE, Kelce WR, Wiese T, et al. Endocrine Screening Methods Workshopreport: detection of estrogenic and androgenic hormonal and antihormonalactivity for chemicals that act via receptor or steroidogenic enzyme mecha-nisms. Reprod Toxicol 1997;11:719–50.
30] Gray Jr LE. Tiered screening and testing strategy for xenoestrogens and antian-drogens. Toxicol Lett 1998;102–103:677–80.
31] Zhang J, Zhang GJ, Zheng YF, Zhu HJ, Yang J, Yao GD, et al. The antiandrogenicactivity of the fungicide N-(3,5-dichlorophenyl) succinimide in in vivo and invitro assays. J Reprod Dev 2007;53:535–43.
32] Sun H, Xu XL, Xu LC, Song L, Hong X, Chen JF, et al. Antiandrogenic activity ofpyrethroid pesticides and their metabolite in reporter gene assay. Chemosphere2007;66:474–9.
33] Kim SS, Lee RD, Lim KJ, et al. Potential estrogenic and antiandrogenic effects ofpermethrin in rats. J Reprod Dev 2005;51:201–10.
34] Wu W, Zhang J, Zhu W, Zheng YF, Zhu HJ, Zhu XQ. The antiandrogenic effects ofcypermethrin and �-cypermethrin. Zhonghua Lao Dong Wei Sheng Zhi Ye BingZa Zhi 2008;26:193–7.
35] Xu LC, Liu L, Ren XM, Zhang MR, Cong N, Xu AQ, et al. Evaluation of andro-gen receptor transcriptional activities of some pesticides in vitro. Toxicology2008;243:59–65.
36] Kunimatsu T, Yamada T, Ose K, Sunami O, KamitaY, OkunoY, et al. Lack ofanti-androgenic or estrogenic effects of three pyrethroids (esfenvalerate, fen-valerate, and permethrin) in the Hershberger and uterotrophic assays. RegulToxicol Pharmacol 2002;35:227–37.
37] Kim HJ, Park YI, Dong MS. Comparison of prostate cancer cell lines forandrogen receptor-mediated reporter gene assays. Toxicol in Vitro 2006;20:1159–67.
38] Coats JR. Mechanisms of toxic action and structure–activity relationships fororganochlorine and synthetic pyrethroid insecticides. Environ Health Perspect1990;87:255–62.
39] Feng J. Development overview of optical activity of pyrethroid insecticide. Pes-ticide 2000;39:1–6 (in Chinese).