S100A6 is secreted from Wharton's jelly mesenchymal stem cells and interacts with integrin β1
Transcript of S100A6 is secreted from Wharton's jelly mesenchymal stem cells and interacts with integrin β1
B
Si
EQ1
N
a
ARR1AA
KCISW
1
b2(aiicla
tbc
mtuPpt
h1
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17
18
19
20
21
22
23
24
25
26
27
28
29
30
31
32
33
34
35
ARTICLE IN PRESSG ModelC 4438 1–6
The International Journal of Biochemistry & Cell Biology xxx (2014) xxx–xxx
Contents lists available at ScienceDirect
The International Journal of Biochemistry& Cell Biology
jo ur nal home page: www.elsev ier .com/ locate /b ioce l
100A6 is secreted from Wharton’s jelly mesenchymal stem cells andnteracts with integrin �1
welina Jurewicz, Agnieszka Góral, Anna Filipek ∗
encki Institute of Experimental Biology, 3 Pasteur Street, 02-093 Warsaw, Poland
r t i c l e i n f o
rticle history:eceived 25 April 2014eceived in revised form0 September 2014ccepted 15 September 2014vailable online xxx
a b s t r a c t
S100A6 is a calcium binding protein belonging to the S100 family. In this work we examined the functionof extracellular S100A6. Using mesenchymal stem cells isolated from Wharton’s jelly of the umbilicalcord (WJMS cells) we have shown that S100A6 is secreted by these cells, and when added to the medium,increases their adhesion and inhibits proliferation. The search for a potential target/receptor of S100A6in the membrane fraction of WJMS cells allowed us to identify some proteins, among them integrin�1, which interacts with S100A6 in a calcium dependent manner. The interaction between S100A6 and
eywords:ell adhesion
ntegrin �1100A6harton’s jelly mesenchymal stem cells
integrin �1, was then confirmed by ELISA using purified proteins. Applying specific antibodies againstintegrin �1 reversed the effect on cell adhesion and proliferation observed in the presence of S100A6which indicates that S100A6 exerts its function due to interaction with integrin �1. Since the data showthe influence of extracellular S100A6 on cells isolated from Wharton’s jelly, our results might help toestablish molecular mechanisms leading to some pathologies characteristic for this tissue.
© 2014 Published by Elsevier Ltd.
36
37
38
39
40
41
42
43
44
45
46
47
48
. Introduction
S100A6 is a low molecular weight calcium binding proteinelonging to the S100 family (Filipek et al., 2008; Lesniak et al.,009). S100A6 expression is high in fibroblasts and epithelial cellsKuznicki et al., 1992), but the protein is also present in neuronalnd glial cells, in smooth muscle and cardiac muscle cells as well asn platelets and lymphocytes. High level of S100A6 is observed inntensively proliferating tumor cells, for instance in melanoma, inhronic and acute myelogenous leukemia or neuroblastoma. Highevel of S100A6 has also been observed in other pathologies suchs liver cirrhosis biliaris or chronic renal disease.
In cells, S100A6 is present mainly in the cytoplasm and exists in
Please cite this article in press as: Jurewicz E, et al. S100A6 is secretedintegrin �1. Int J Biochem Cell Biol (2014), http://dx.doi.org/10.1016/j
he form of non-covalent dimers. Each S100A6 monomer is able toind Ca2+ through the “EF-hand” motifs. Binding of Ca2+ inducesonformational changes in the S100A6 molecule which allow
Abbreviations: AGE, advanced glycation end products; BSA, bovine serum albu-in; DPBS, Dulbecco’s phosphate buffered saline; DTT, dithiotreitol; GST, glu-
athione-S-transferase; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazoli-m bromide; PBS, phosphate buffered saline; PBS-T, PBS containing 0.05% Tween20;MSF, phenylmethylsulfonyl fluoride; RAGE, receptor for advanced glycation endroducts; SDS, sodium dodecyl sulfate; TLR, toll-like receptor; WJMS cells, Whar-on’s jelly mesenchymal stem cells.∗ Corresponding author. Tel.: +48 22 589 23 32; fax: +48 22 822 53 42.
E-mail address: [email protected] (A. Filipek).
Q2
ttp://dx.doi.org/10.1016/j.biocel.2014.09.015357-2725/© 2014 Published by Elsevier Ltd.
49
50
51
52
53
54
55
56
57
58
interaction with target proteins. The established S100A6 interac-tions with ligands indicate that this protein might play a role invarious cellular processes such as regulation of actin cytoskele-ton organization or differentiation (Tonini et al., 1991). S100A6has also been found extracellularly, for instance in pancreatic juice,where its presence might have a diagnostic importance in pancre-atic cancer (Ohuchida et al., 2007), in the amnion fluid (Celis et al.,1990) and in the medium of cultured decidual cells (Thordarsonet al., 1991). It is noteworthy that S100A6 added to the culture oftrophoblast cells increased lactogen II secretion. It has been alsoshown that S100A6 added extracellularly increases the release ofinsulin from pancreatic � cells (Okazaki et al., 1994) and decreasesthe release of histamine from mast cells (Fujii et al., 1994). Sub-sequent studies have shown that S100A6 binds to the receptorfor advanced glycation end products (RAGE) and, moreover, thatthe S100A6-RAGE interaction might modulate apoptosis/survival
of neuroblastoma SH-SY5Y cells (Leclerc et al., 2007; Donato, 2007).Our recent work has shown that S100A6 is present in Whar-
ton’s jelly of umbilical cord (Jurewicz et al., 2012). Wharton’s jellyconsists mainly of extracellular matrix and fibroblast-like cells(McElreavey et al., 1991). These cells, called mesenchymal stemcells (MSC) or Wharton’s jelly mesenchymal stem cells (WJMS cells)(Taghizadeh et al., 2011; Fong et al., 2009; Wang et al., 2004) were
from Wharton’s jelly mesenchymal stem cells and interacts with.biocel.2014.09.015
used in our studies to elucidate extracellular function of S100A6 inWharton’s jelly. At first we examined whether S100A6 is secretedby WJMS cells and then we checked the effect of extracellular
59
60
61
ING ModelB
2 of Bioc
Sff
2
2
jfcMtcSkocnca1alSbtcof
2s
CmAbfEt5ifnt1sd4sP
2
c(tN1taf
62
63
64
65
66
67
68
69
70
71
72
73
74
75
76
77
78
79
80
81
82
83
84
85
86
87
88
89
90
91
92
93
94
95
96
97
98
99
100
101
102
103
104
105
106
107
108
109
110
111
112
113
114
115
116
117
118
119
120
121
122
123
124
125
126
127
128
129
130
131
132
133
134
135
136
137
138
139
140
141
142
143
144
145
146
147
148
149
150
151
152
153
154
155
156
157
158
159
160
161
162
163
164
165
166
167
168
169
170
171
172
173
174
175
176
ARTICLEC 4438 1–6
E. Jurewicz et al. / The International Journal
100A6 on properties of these cells. In the next step we lookedor a receptor on the plasma membrane of WJMS cells responsibleor transducing the effects of extracellular S100A6.
. Materials and methods
.1. Cell culture, SDS-PAGE and immunoblotting
Umbilical Cord-Derived Mesenchymal Stem Cells (Wharton’selly mesenchymal stem cells, called WJMS cells) were obtainedrom ATCC and cultured according to the manufacturer proto-ol in Mesenchymal Stem Cell Basal Medium supplemented withesenchymal Stem Cell Growth Kit, penicillin (10 U/ml) and strep-
omycin (0.1 mg/ml) in 5% CO2 at 37 ◦C. Cells were passaged whenonfluent and used for experiments 24 h later. For analysis of100A6 secretion, 80% confluent cells were washed in DPBS andept in Mesenchymal Stem Cell Basal Medium (with 2% FBS) with-ut antibiotics for 4 h at 37 ◦C in 5% CO2. Then the medium wasollected and centrifuged at 16,000 × g for 15 min at 4 ◦C. Super-atant (400 �l) was concentrated by acetone precipitation. Forontrol, 400 �l of culture medium incubated on empty plate for 4 ht 37 ◦C in 5% CO2 was used. Precipitated proteins were subjected to5% polyacrylamide gel, performed according to Laemmli (1970),nd then transferred electrophoretically onto nitrocellulose. Theevel of S100A6 was estimated using polyclonal antibodies against100A6 (produced in-house and checked for specificity as describedy Filipek et al., 1993) diluted 1:500. Then, the blots were allowedo react with goat anti-rabbit IgG (Sigma) secondary antibodiesonjugated to horseradish peroxidase, diluted 1:10,000 and devel-ped with the ECL chemiluminescence kit (Amersham Biosciences)ollowed by exposure against an X-ray film.
.2. Affinity chromatography/pull-down assay and masspectrometry analysis
The coupling of purified recombinant S100A6 protein toNBr-activated-Sepharose (Fluka) was carried out according toanufacturer’s instructions as described by Slomnicki et al. (2009).t the same time a control resin with no coupled protein, butlocked with Tris, was prepared. The plasma membrane fractionrom WJMS cells was obtained using the Plasma Membrane Proteinxtraction Kit (Abcam) according to the manufacturer’s instruc-ion and then was diluted in buffer containing 20 mM Tris pH 7.5,0 mM NaCl, 1 mM DTT, 2 mM CaCl2, 1% Nonidet and protease
nhibitors (protease inhibitor cocktail; Roche). 150 �g of proteinsrom this fraction were applied to the control resin (to eliminateon-specific binding) and then the unbound fraction was appliedo S100A6-CNBr-Sepharose and incubated on a rotating wheel for
h at 4 ◦C. The resin was extensively washed with the above bufferupplemented with 1 M NaCl and then proteins bound in a Ca2+-ependent manner were eluted with buffer as above but containing
mM EGTA, precipitated with cold acetone and subjected to masspectrometry (Institute of Biochemistry and Biophysics, Warsaw,oland).
.3. Immunofluorescence
Immunofluorescence staining was performed on WJMS cellsultured on glass cover slips previously coated with poly-l-lysine50 �g/ml) for 24 h. Then the coverslips were placed on ice andhe medium was replaced by ice-cold buffer containing 10 mMaHCO3, 10 mM glucose, 5 mM KCl, 125 mM NaCl, 1 mM CaCl2,
Please cite this article in press as: Jurewicz E, et al. S100A6 is secretedintegrin �1. Int J Biochem Cell Biol (2014), http://dx.doi.org/10.1016/j
mM MgCl2 and 20 mM Hepes pH 7.5. Cells were incubated withhe above buffer for 10 min on ice and then S100A6 was added to
final concentration of 1 �M and incubation on ice was prolongedor 30 min. Then the coverslips were treated with 50 mM NH4Cl for
PRESShemistry & Cell Biology xxx (2014) xxx–xxx
10 min at room temperature in order to reduce aldehyde groups.In the next step cells were incubated in TBS containing 3% of BSAand 1 mM CaCl2 for 45 min on ice and subsequently for 1 h on icewith anti-S100A6 rabbit polyclonal antibody (produced in-house)and anti-integrin �1 mouse monoclonal antibody (Abcam) diluted1:50 in the same buffer. After washing the cells were fixed with2% paraformaldehyde in buffer containing 120 mM PIPES, 50 mMHepes, 8 mM MgCl2 and 1 mM CaCl2, pH 6.9 for 10 min on ice andthen for 20 min at room temperature. In the next step the reactionwith secondary antibodies was carried out at room temperaturefor 40 min, using anti-rabbit Alexa Fluor 488 and anti-mouse AlexaFluor 555 (Molecular Probes) diluted 1:200 in TBS with 3% of BSAand 1 mM CaCl2. After final washing with TBS containing 1 mMCaCl2 and subsequently with water, the coverslips were mountedon slides with VectaShield containing DAPI (Vector Laboratories).Immunofluorescence staining was analyzed under a Leica micro-scope (TCS SP8).
2.4. Proteins
The S100A6 protein for preparation of the affinity resin and forother experiments was purified as described by Slomnicki et al.,2009. GST protein, used as control, was purified on GlutathioneSepharose 4B (GE Healthcare), following the procedure outlined bythe manufacturer. Recombinant human proteins: full length inte-grin �1 and RAGE, both with GST tags were purchased from Abcam,plexin B2 form R&D Systems and vasorin from Novoprotein.
2.5. ELISA assay
Integrin �1, plexin B2, vasorin, GST or BSA (400 ng/well each)were coated onto a 96-well microtiter plate in 50 �l of coat-ing buffer containing 100 mM Na2HPO4, 100 mM NaH2PO4, pH8.1. After overnight incubation at 4 ◦C, the solution was removedand the wells were washed four times with PBS-T. The remain-ing adsorption sites were blocked for 3 h at room temperatureusing PBS containing 10% BSA. Then, increasing amounts of S100A6were added in 100 �l of reaction buffer containing 10 mM Tris pH7.5, 50 mM NaCl, 5% glycerol, 1 mg/ml BSA, 1 mM CaCl2 or 2 mMEGTA. After overnight incubation at 4 ◦C, the solution was removed.Following the washing procedure with PBS-T, rabbit polyclonalantibodies against S100A6 (produced in-house) diluted 1:500 inPBS containing 10% BSA were added and incubation was carriedout for 3 h at room temperature. This was followed by addition ofsecondary goat anti-rabbit IgG antibodies (Sigma) diluted 1:7000in PBS containing 10% BSA. After washing, the analysis of boundantibodies was performed by colorimetric detection with TMB per-oxidase EIA substrate kit (BioRad). The reaction was stopped with100 �l of 1 M sulfuric acid and the absorbance at 450 nm was mea-sured using a Bio-Rad microplate reader.
In the ELISA assay performed to compare the binding of S100A6with integrin �1 and with RAGE the wells were coated with equalmolar amounts of integrin �1 or RAGE and then different amountsof S100A6 were added.
2.6. Cell adhesion assay
The effect of S100A6 on adhesion of WJMS cells was analyzedby a colorimetric assay using MTT. For that wells were coatedovernight at 4 ◦C with fibronectin (15 �g/ml) and then blocked with0.4% BSA in PBS for 2 h. Prior to experiments cells were detachedusing PBS with 0.05% EDTA and counted in the Neubauer chamber.
from Wharton’s jelly mesenchymal stem cells and interacts with.biocel.2014.09.015
Then, 5 × 103 cells were incubated with S100A6 (10 nM, 100 nM or1 �M) for 20 min at 37 ◦C. Cells were seeded at the density of 5 × 103
per well in Mesenchymal Stem Cell Basal Medium. Following incu-bation for 1 h at 37 ◦C, wells were vigorously washed 3 times with
177
178
179
180
IN PRESSG ModelB
of Biochemistry & Cell Biology xxx (2014) xxx–xxx 3
Dfas
gaa(aca
2
Nob12t
Wia(CiptwwipbPBct5aaVr(sp
3
3e
wpi1of
aew
Fig. 1. Secretion of S100A6 from WJMS cells analyzed by immunoblotting using spe-cific polyclonal antibodies against this protein. (1) Control medium and (2) mediumtaken from WJMS cells cultured for 4 h. In each case 400 �l of medium was concen-trated by acetone precipitation, applied on SDS polyacrylamide gel and analyzed byWestern blot.
Fig. 2. Adhesion of WJMS cells in the presence of S100A6. Cells were incubatedfor 20 min in the presence of different concentrations (10 nM – gray bar, 100 nM –dark gray bar or 1 �M – black bar) of S100A6 and then cultured for 1 h. Analysis ofdata from four independent experiments is presented as a mean ± SEM; *p ≤ 0.05,**p≤0.01.
Fig. 3. Proliferation of WJMS cells in the presence of different concentrations ofS100A6. Cells were incubated with S100A6 and then counted after the time indicatedin the figure. Black bars–cells incubated without S100A6 (control). Gray, dotted and
181
182
183
184
185
186
187
188
189
190
191
192
193
194
195
196
197
198
199
200
201
202
203
204
205
206
207
208
209
210
211
212
213
214
215
216
217
218
219
220
221
222
223
224
225
226
227
228
229
230
231
232
233
234
235
236
237
238
239
240
241
242
243
244
245
246
247
248
249
250
251
252
253
254
255
256
257
258
259
260
ARTICLEC 4438 1–6
E. Jurewicz et al. / The International Journal
PBS and then MTT was added. After incubation for 3 h at 37 ◦Collowed by 3 washes with DPBS, DMSO was added. Absorbancet 570 nm was measured using a Bio-Rad microplate reader andtatistical data analysis was performed using Student’s t test.
The effect of S100A6 on cell adhesion after blocking inte-rin �1 was analyzed as described above but prior to S100A6ddition 5 × 103 cells were incubated with 15 �g/ml monoclonalnti-integrin �1 antibody (Abcam), with 15 �g/ml anti-mouse IgGInvitrogen) or with PBS (these two last served as control) for 20 mint 37 ◦C. Then cells were incubated with S100A6 at a final con-entration of 1 �M for 20 min at 37 ◦C and treated as describedbove.
.7. Proliferation assay
To check the proliferation rate, WJMS cells were counted in theeubauer chamber and plated onto 48-well dishes at the densityf 5 × 103 cells per well. After 24 h growth, medium was replacedy medium containing different concentrations of S100A6 (10 nM,00 nM or 1 �M) and without this protein (control). Then, after4 h, 48 h and 72 h cells were counted and the results normalizedo values obtained with cells not exposed to S100A6.
To prove that extracellular S100A6 reduces proliferation ofJMS cells, a BrdU assay was performed. In order to neutralize
ntegrin �1 or RAGE, cells were incubated with rabbit polyclonalnti-integrin �1 (Abcam) or goat polyclonal anti-RAGE antibodiesSanta Cruz) at a final concentration of 15 �g/ml for 20 min at 37◦
. Then, S100A6 was added at a final concentration of 1 �M andncubation was carried out for 20 min at 37◦ C. Next, cells werelated onto coverslips. After 48 h, BrdU reagent, at a final concen-ration of 10 �M, was added for 24 h. Finally, 72 h after seeding, cellsere fixed with 70% ethanol in −20 ◦C for 24 h. Subsequently, cellsere washed 2 × 5 min with PBS containing 0.5% Triton X100 and
ncubated with PBS containing 2 M HCl for 30 min at room tem-erature. After washing with PBS cells were treated with 0.1 Morax for 1 min at room temperature, then washed again withBS and incubated for 1 h at room temperature with mouse anti-rdU antibody (BD Transduction Laboratories) diluted 1:50 in PBSontaining 1% BSA and 0.5% Tween20. After washing with PBS con-aining 0.5% Tween20 secondary anti-mouse antibody Alexa Fluor55 (Molecular Probes) diluted 1:500 in PBS containing 1% BSAnd 0.5% Tween20, was added for 1 h at room temperature. Then,fter final washing, the coverslips were mounted on slides withectaShield containing DAPI (Vector Laboratories). Immunofluo-escence staining was analyzed under a fluorescence microscopeZeiss Observer.Z1). Counting was performed using the ImageJoftware and the number of BrdU labeled cells was calculated asercentage of DAPI positive cells.
. Results
.1. Secretion of S100A6 from WJMS cells and influence ofxtracellular S100A6 on cell adhesion and proliferation
To check whether S100A6 is secreted, medium from WJMS cellsas concentrated by acetone precipitation and proteins from theellet were separated in polyacrylamide gel and then analyzed by
mmunoblotting. As it is shown in Fig. 1, a band at the level of about0 kD, corresponding to the molecular weight of S100A6, was rec-gnized by specific anti-S100A6 antibody in the medium recoveredrom the cell culture but not from the control medium.
Please cite this article in press as: Jurewicz E, et al. S100A6 is secretedintegrin �1. Int J Biochem Cell Biol (2014), http://dx.doi.org/10.1016/j
Since S100A6 is secreted by WJMS cells in the next step wenalyzed whether S100A6 added to cultured medium had anyffect on cell adhesion and proliferation. To check that, WJMS cellsere incubated with different concentrations of S100A6 and then
white bars – cells incubated with 10 nM, 100 nM and 1 �M of S100A6, respectively.Data from three independent experiments are presented as a mean ± SEM; *p ≤ 0.05,**p ≤ 0.01, ***p ≤ 0.001.
adhesion of these cells was monitored by a colorimetric assay usingMTT. As it is seen in Fig. 2, in the presence of S100A6 cell adhesionis higher. Concentrations of S100A6 of about 100 nM and 1 �M aresufficient to obtain an effect which is statistically significant. Since,usually, an increase in cell adhesion is correlated with decrease inproliferation, WJMS cells were incubated with different concen-trations of S100A6 and the proliferation rate was monitored. Asillustrated in Fig. 3, proliferation of cells incubated with S100A6 isdecreased.
3.2. Identification of membrane receptor of S100A6
To learn more about the mechanism responsible for transducingthe effect of extracellularly added S100A6 we searched for its mem-brane ligand/receptor. For that membrane fraction from WJMS cellswas obtained and then proteins were applied on S100A6 affin-ity resin in buffer containing Ca2+. Proteins which were bound inthe presence of Ca2+ and eluted in buffer containing EGTA wereanalyzed by mass spectrometry. As it is shown in Table 1, threemembrane proteins, integrin �1, plexin B2 and vasorin, were iden-tified by this method to be potential membrane targets of S100A6.To confirm interaction of S100A6 with these targets ELISA assays
from Wharton’s jelly mesenchymal stem cells and interacts with.biocel.2014.09.015
were performed using purified proteins. As it could be seen inFig. 4A, the interaction between S100A6 and plexin B2 or vasorinwere very weak. In contrast, the binding of S100A6 to integrin�1 was much stronger and depended on the presence of calcium
261
262
263
264
ARTICLE IN PRESSG ModelBC 4438 1–6
4 E. Jurewicz et al. / The International Journal of Biochemistry & Cell Biology xxx (2014) xxx–xxx
Table 1Potential targets of S100A6 in membrane fraction of WJMS cells identified by massspectrometry.
NCBI Accession Number Proteins
gi|2280476 Plexin B2gi|179955 Catechol O-methyltransferase (COMT)gi|19743813 Integrin �1gi|177207 4F2 antigen heavy chaingi|15489339 Vasorin
Fig. 4. ELISA assay showing the binding of S100A6 to membrane proteins. (A) ELISAassay showing the binding of S100A6 to plexin B2 and vasorin. (B) ELISA assay show-ing the binding of S100A6 to integrin �1. 400 ng/well of each recombinant proteinwas immobilized onto ELISA plate wells. Binding of S100A6 to vasorin or integrin�1 in the presence of Ca2+ (black diamonds) or in the presence of EGTA (white dia-monds). Binding of S100A6 to plexin B2 or GST in the presence of Ca2+ (black squares)ooe
(GAGiRwSaos
tfasabTtFsiii
Fig. 5. ELISA assay showing the binding of S100A6 to RAGE and integrin �1.400 ng/well of integrin �1, 190 ng/well of RAGE or 190 ng/well of GST were immobi-lized onto ELISA plate and then increasing amounts of S100A6 (from 100 to 700 nM)were added. Binding of S100A6 to integrin �1 in the presence of Ca2+ (black dia-monds) or in the presence of EGTA (white diamonds). Binding of S100A6 to RAGE(black triangles) or in the presence of EGTA (white triangles), binding of S100A6to GST in the presence of Ca2+ (black squares) or in the presence of EGTA (white
265
266
267
268
269
270
271
272
273
274
275
276
277
278
279
280
281
282
283
284
285
286
287
288
289
290
291
292
293
294
295
296
297
298
299
300
301
302
303
304
305
306
307
308
309
310
311
312
313
314
315
316
317
318
319
320
321
322
323
324
325
326
327
328
r in the presence of EGTA (white squares). Binding of S100A6 to BSA in the presencef Ca2+ (black circles) or in the presence of EGTA (white circles). A representativexperiment out of three performed is shown.
Fig. 4B). Since commercial integrin �1 was available only with aST tag the interaction between S100A6 and GST was also checked.s it can be seen in Fig. 4B there is no binding between S100A6 andST or BSA which means that the S100A6-integrin �1 interaction
s specific. Since it has been published that S100A6 interacts withAGE the binding between S100A6-RAGE, and S100A6-integrin �1as compared. As it can be seen in Fig. 5 the interaction between
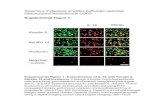
100A6 and integrin �1 is much stronger than between S100A6nd RAGE. Moreover, the immunofluorescence staining performedn WJMS cells showed that S100A6 and integrin �1 co-localized inome areas of the plasma membrane of these cells (Fig. 6).
To check whether S100A6 might have an effect on cell adhesionhrough interaction with integrin �1, an adhesion assay was per-ormed as described above but cells were incubated with specificnti-integrin �1 antibody prior to S100A6 addition. The results pre-ented in Fig. 7 show that adhesion of WJMS cells incubated withnti-integrin �1 was decreased. Addition of S100A6 to cells incu-ated with anti-integrin �1 antibody did not increase cell adhesion.o prove that S100A6 might have an effect on cell proliferationhrough interaction with integrin �1, a BrdU assay was performed.or that, integrin �1 or RAGE were neutralized by incubation with
Please cite this article in press as: Jurewicz E, et al. S100A6 is secretedintegrin �1. Int J Biochem Cell Biol (2014), http://dx.doi.org/10.1016/j
pecific antibodies prior to S100A6 addition. The results presentedn Fig. 8 show that proliferation of WJMS cells incubated with anti-ntegrin �1 antibody increased in the presence of S100A6. However,n the case when antibodies against RAGE were applied there was
squares) and binding of S100A6 to BSA in the presence of Ca2+ (black circles) orin the presence of EGTA (white circles). A representative experiment out of threeperformed is shown.
no difference in the number of BrdU positive cells in the presenceor absence of S100A6. The obtained results confirm that the effectof S100A6 on WJMS cells adhesion and proliferation is due to itsbinding to integrin �1.
4. Discussion
We have shown previously that S100A6 is present in extracellu-lar matrix of Wharton’s jelly of the umbilical cord (Jurewicz et al.,2012, 2014). To examine the function of extracellular S100A6, inthis work we used mesenchymal stem cells isolated from Whar-ton’s jelly (WJMS cells). We have shown that S100A6 is secretedby these cells. Up to now, it has been reported that S100A6 ispresent in pancreatic juice (Ohuchida et al., 2007), in the amnionfluid (Celis et al., 1990) and in the medium of cultured decidualcells (Thordarson et al., 1991). Thus, in this manuscript secretionof S100A6 from WJMS cells has been shown for the first time. Inthe next step we checked the effect of extracellular S100A6 on theproperties of WJMS cells. We have found that extracellular S100A6increases adhesion and inhibits proliferation of these cells. It hasbeen published earlier that when added to the cell medium S100A6caused (1) an increase in secretion of lactogen II from trophoblastcells, (2) an increase in the release of insulin from pancreatic �cells and (3) a decrease in the release of histamine from mast cells(reviewed in Lesniak et al., 2009). Thus, results presented in thismanuscript show for the first time the influence of extracellularS100A6 on cell adhesion and proliferation. All data regarding thesecretion of S100A6 and its effect on certain aspects of cell phys-iology clearly indicate that S100A6 exerts its biological functionthrough binding to a specific membrane receptor.
Earlier studies regarding the search for the possible receptorhave shown that S100A6 binds to RAGE (receptor for advancedglycation end products) and that the S100A6-RAGE interactionmight modulate apoptosis/survival of neuroblastoma SH-SY5Ycells (Leclerc et al., 2007). However, these effects were observedat relatively high concentrations of extracellular S100A6 (Donatoet al., 2013). Moreover, RAGE was shown to be a receptor not onlyfor S100A6 but also for several other proteins from the S100 fam-ily namely S100B, S100A1, S100A4, S100A5, S100A7, S100A8/9,S100A11, S100A12, S100A13 and S100P (reviewed in Leclerc et al.,2009). Regarding S100-RAGE interaction, it is known that various
from Wharton’s jelly mesenchymal stem cells and interacts with.biocel.2014.09.015
S100 proteins might bind to different sites on RAGE. For instance,S100B binds strictly to the V domain, S100A6 can bind to the V-and C2-domains and S100A12 to the V- and C1-domains (Leclercet al., 2009). It should be also underlined that other members of the
329
330
331
332
ARTICLE IN PRESSG ModelBC 4438 1–6
E. Jurewicz et al. / The International Journal of Biochemistry & Cell Biology xxx (2014) xxx–xxx 5
Fig. 6. Co-localization of S100A6 and integrin �1 on WJMS cell membrane.InS
Sebewa2
Fig. 7. Influence of anti-integrin �1 antibody on adhesion of WJMS cells in the pres-ence of S100A6 at 1 �M concentration. White bar – cells incubated with S100A6, graybar – cells incubated with anti-mouse IgG antibody and S100A6, dotted bar – cellsincubated with anti-integrin �1 specific antibody and black bar - cells incubatedwith anti-integrin �1 specific antibody and S100A6. Analysis of data from threeindependent experiments is presented as a mean ± SEM; **p ≤ 0.01, ***p ≤ 0.001.
Fig. 8. Influence of anti-integrin �1 and anti-RAGE antibodies on proliferation ofWJMS cells in the absence or presence of 1 �M S100A6. White bar – cells incu-bated with S100A6 alone (control). Lined bars represent cells preincubated withspecific anti-integrin �1 antibody without (vertical lines) or with (diagonal lines)subsequent addition of S100A6. Plain bars represent cells preincubated with specificanti-RAGE antibody without (gray) or with (black) subsequent addition of S100A6.
333
334
335
336
337
338
339
340
341
342
343
344
345
346
347
348
349
350
351
352
353
354
355
356
357
358
359
360
361
362
mmunofluorescence staining of S100A6 is seen in green and of integrin �1 in red;uclei stained with DAPI are seen in blue. The yellow color in merge indicates that100A6 co-localizes with integrin �1. The scale bar is 5 �m.
100 family bind to membrane proteins different than RAGE. Suchxamples are S100A4 and S100B which were shown to functiony activating EGFR and EGFR1 receptors, respectively (Klingelhöfer
Please cite this article in press as: Jurewicz E, et al. S100A6 is secretedintegrin �1. Int J Biochem Cell Biol (2014), http://dx.doi.org/10.1016/j
t al., 2009; Riuzzi et al., 2011). Another example is S100A9 whichas shown to mediate neutrophil adhesion to fibronectin through
ctivation of integrin �2 (Newton and Hogg, 1998; Anceriz et al.,007). Quite recently, it has been reported that S100A8 and S100A9
363
The percentage of BrdU positive cells is presented as a mean ± SEM; *p < 0.05,**p < 0.01, ***p < 0.001.
might activate the innate immune system via interaction with TLRs(Tool-like receptors) (Ieguchi et al., 2013; Tong et al., 2014) whileS100A1, via transient activation of TLR4, might modulate myocar-dial wound healing (Rohde et al., 2014). Also, it has been shownthat the interaction of S100A9 with CD85j receptor is implicated inthe control of HIV-1 replication by NK cells (Arnold et al., 2013).
To examine whether S100A6 acts on WJMS cells through inter-action with RAGE or through another membrane receptor weanalyzed by mass spectrometry proteins from the membrane frac-tion that bound to S100A6 affinity resin in the presence of Ca2+.Interestingly, not RAGE but some other membrane proteins werefound to interact with S100A6. Among them were plexin B2, vasorinand integrin �1. Direct interactions between S100A6 and theseproteins were then analyzed by ELISA using purified proteins.Interestingly, the strongest interaction was found for S100A6 andintegrin �1. Integrins belong to a family of glycosylated, type Itransmembrane receptors that consist of non-covalently bound �and � subunits (Van der Flier and Sonnenberg, 2001; Jurewicz et al.,2014). The �1 subunit is expressed by most cell types and medi-ates predominantly cell-matrix adhesion. To check whether indeedthe effect exerted by extracellular S100A6 on WJMS cells is due toS100A6-integrin �1 interaction we analyzed adhesion and prolif-eration in the presence of specific antibodies against integrin �1. Asexpected, applying anti-integrin �1 antibodies reversed the effect
from Wharton’s jelly mesenchymal stem cells and interacts with.biocel.2014.09.015
caused by S100A6. At present the molecular mechanism of S100A6action on integrin �1 is not known. One can speculate that S100A6either facilitates oligomerization of integrin �1 or might cross-link
364
365
366
ING ModelB
6 of Bioc
in
taifhadi
A
trERQ3sQ4f
R
A
A
C
D
D
F
F
F
F
Q5
367
368
369
370
371
372
373
374
375
376
377
378
379
380
381
382
383
384
385
386
387
388
389
390
391
392
393
394
395
396
397
398
399
400
401
402
403
404
405
406
407
408
409
410
411
412
413
414
415
416
417
418
419
420
421
422
423
424
425
426
427
428
429
430
431
432
433
434
435
436
437
438
439
440
441
442
443
444
445
446
447
448
449
450
451
452
453
454
455
456
457
458
459
460
461
462
463
464
465
466
467
ARTICLEC 4438 1–6
E. Jurewicz et al. / The International Journal
ntegrin �1 to fibronectin. To resolve this issue further studies areeeded.
Altogether, data presented in this manuscript show for the firstime that extracellular S100A6, at least in some types of cells, mightct through a different receptor than RAGE. Thus, results presentedn this work extend our knowledge regarding membrane ligandsor S100 proteins and indicate that particular S100 proteins mightave different receptors depending on the cell type. Since WJMSre regarded to be of potential use in future cell based therapies,etermination of the role of S100A6-mediated signaling pathway
n these cells is of great importance.
cknowledgments
We thank Prof. K. Kwiatkowska (Nencki Institute of Experimen-al Biology, Poland) for her suggestions regarding immunofluo-escence analysis and Professors W. Lesniak (Nencki Institute ofxperimental Biology, Poland) and Y. Wegrowski (University ofeims, France) for critical reading of the manuscript. This work wasupported by NCN grant (NZ4/01664) to A.F. and by statutory fundsrom the Nencki Institute of Experimental Biology.
eferences
nceriz N, Vandal K, Tessier PA. S100A9 mediates neutrophil adhesion tofibronectin through activation of beta2 integrins. Biochem Biophys Res Commun2007;354:84–9.
rnold V, Cummings JS, Moreno-Nieves UY, Didier C, Gilbert A, Barré-Sinoussi F,et al. S100A9 protein is a novel ligand for the CD85j receptor and its interac-tion is implicated in the control of HIV-1 replication by NK cells. Retrovirology2013;10:122.
elis JE, Crüger D, Kiil J, Dejgaard K, Lauridsen JB, Ratz GP, et al. A two dimensional gelprotein database of noncultured total normal human epidermal keratinocytes:identification of proteins strongly upregulated in psoriatic epidermis. Elec-trophoresis 1990;11:242–54.
onato R, Cannon BR, Sorci G, Riuzzi F, Hsu K, Weber DJ, et al. Functions of S100proteins. Curr Mol Med 2013;13:24–57.
onato R. RAGE: a single receptor for several ligands and different cellular responses:the case of certain S100 proteins. Curr Mol Med 2007;7:711–24.
ilipek A, Michowski W, Kuznicki J. Involvement of S100A6 (calcyclin) andits binding partners in intracellular signaling pathways. Adv Enzyme Regul2008;48:225–39.
ilipek A, Puzianowska M, Cieslak B, Kuznicki J. Calcyclin – Ca(2+)-binding pro-tein homologous to glial S-100 beta is present in neurones. Neuroreport1993;4:383–6.
ong CY, Gauthaman K, Bongso A. Reproductive stem cells of embryonic origin:comparative properties and potential benefits of human embryonic stem cells
Please cite this article in press as: Jurewicz E, et al. S100A6 is secretedintegrin �1. Int J Biochem Cell Biol (2014), http://dx.doi.org/10.1016/j
and wharton’s jelly stem cells; stem cells in human reproduction. 2nd ed.; 2009.p. 272.
ujii T, Kuzumaki N, Ogoma Y, Kondo Y. Effects of calcium-binding proteins on his-tamine release from permeabilized rat peritoneal mast cells. Biol Pharm Bull1994;17:581–5.
PRESShemistry & Cell Biology xxx (2014) xxx–xxx
Ieguchi K, Omori T, Komatsu A, Tomita T, Deguchi A, Maru Y. Ephrin-A1 expressioninduced by S100A8 is mediated by the toll-like receptor 4. Biochem Biophys ResCommun 2013;440:623–9.
Jurewicz E, Kasacka I, Bankowski E, Filipek A. S100A6 and its extracellulartargets in Wharton’s jelly of healthy and preeclamptic patients. Placenta2014;35:386e391.
Jurewicz E, Kasacka I, Bankowski E, Filipek A. Identification and localization ofS100A6 in human umbilical cord. Cell Biol Int 2012;36:109–12.
Klingelhöfer J, Møller HD, Sumer EU, Berg CH, Poulsen M, Kiryushko D, et al. Epi-dermal growth factor receptor ligands as new extracellular targets for themetastasis-promoting S100A4 protein. FEBS J 2009;276:5936–48.
Kuznicki J, Kordowska J, Puzianowska M, Wozniewicz BM. Calcyclin as amarker of human epithelial cells and fibroblasts. Exp Cell Res 1992;200:425–30.
Laemmli UK. Cleavage of structural proteins during the assembly of the head ofbacteriophage T4. Nature 1970;227:680–5.
Leclerc E, Fritz G, Vetter SW, Heizmann CW. Binding of S100 proteins to RAGE: anupdate. Biochim Biophys Acta 2009;1793:993–1007.
Leclerc E, Fritz G, Weibel M, Heizmann CW, Galichet A. S100B and S100A6 dif-ferentially modulate cell survival by interacting with distinct RAGE (receptorfor advanced glycation end products) immunoglobulin domains. J Biol Chem2007;282:31317–31.
Lesniak W, Słomnicki ŁP, Filipek A. S100A6-new facts and features. Biochem BiophysRes Commun 2009;390:1087–92.
McElreavey KD, Irvine AI, Ennis KT, McLean WH. Isolation, culture and characterisa-tion of fibroblast-like cells derived from the Wharton’s jelly portion of humanumbilical cord. Biochem Soc Trans 1991;19:29S.
Newton R, Hogg NJ. The human S100 protein MRP-14 is a novel activator of the beta2 integrin Mac-1 on neutrophils. J Immunol 1998;160:1427–35.
Ohuchida K, Mizumoto K, Yu J, Yamaguchi H, Konomi H, Nagai E, et al. S100A6is increased in a stepwise manner during pancreatic carcinogenesis: clinicalvalue of expression analysis in 98 pancreatic juice samples. Cancer EpidemiolBiomarkers Prev 2007;16:649–54.
Okazaki K, Niki I, Iino S, Kobayashi S, Hidaka H. A role of calcyclin, a Ca(2+)-bindingprotein, on the Ca(2+)-dependent insulin release from the pancreatic beta cell.J Biol Chem 1994;269:6149–52.
Riuzzi F, Sorci G, Donato R. S100B protein regulates myoblast proliferation anddifferentiation by activating FGFR1 in a bFGF-dependent manner. J Cell Sci2011;124:2389–400.
Rohde D, Schön C, Boerries M, Didrihsone I, Ritterhoff J, Kubatzky KF, et al. S100A1is released from ischemic cardiomyocytes and signals myocardial damage viaToll-like receptor 4. EMBO Mol Med 2014;6:778–94.
Slomnicki L, Nawrot B, Lesniak W. S100A6 binds p53 and effects its activity. Int JBiochem Cell Biol 2009;41:784–90.
Taghizadeh RR, Cetrulo KJ, Cetrulo CL. Wharton’s Jelly stem cells: future clinicalapplications. Placenta 2011;4:S311–5.
Thordarson G, Southard JN, Talamantes F. Purification and characterization of mousedecidual calcyclin: a novel stimulator of mouse placental lactogen-II secretion.Endocrinology 1991;129:1257–65.
Tong L, Lan W, Lim RR, Chaurasia SS. S100A proteins as molecular targets in theocular surface inflammatory diseases. Ocul Surf 2014;12:23–31.
Tonini GP, Casalaro A, Cara A, Di Martino D. Inducible expression of calcyclin, agene with strong homology to S-100 protein, during neuroblastoma cell dif-ferentiation and its prevalent expression in Schwann-like cell lines. Cancer Res
from Wharton’s jelly mesenchymal stem cells and interacts with.biocel.2014.09.015
1991;51:1733–7.Van der Flier A, Sonnenberg A. Function and interactions of integrins. Cell Tissue Res
2001;305:285–98.Wang HS, Hung SC, Peng ST, Huang CC, Wei HM, Guo YJ, et al. Mesenchymal stem cells
in the Wharton’s jelly of the human umbilical cord. Stem Cells 2004;22:1330–7.
468
469
470
471
472







![University of Western Australia · Web view, as it helps to completely degrade chitin degradation products, generated by secreted chitinases [43,44] and transported through outer](https://static.fdocument.org/doc/165x107/60d97f7be5724d3db967093f/university-of-western-australia-web-view-as-it-helps-to-completely-degrade-chitin.jpg)