PRODUCTION
Transcript of PRODUCTION

PRODUCTION
NITRIC ACID
CRUSH and GRIND REDUCE • $ >
r LEACH
ACID RECOVERY
DECOMPOSE
1 EVAPORATE
FILTER
Τ WASH - • \ DRY / ^âilAJUNG
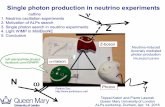
Nossen Labs' new manganese concentration process uses a nitric acid cycle, produces iron and calcium nitrate as by-products
Manganese — Off the Critical List? New ore concentration process uses domestic sup
plies from Maine, may free U. S. foreign sources
C R I T I C A L MANGANESE may get a boost ^ from a new concentration process pilot-planted in Paterson, N. J. At the rate of 1000 pounds of ore every hour, plant produces a concentrate containing 60% manganese, recovering 80% of the metal from low grade ores available in the U. S. Pilot plant has been operating all summer; concentrate gives every indication of competing successfully with imported high grade ores.
About 90% of U . S. manganese ore is imported, from India, Africa, South America, even Russia. Every ton of steel produced requires 13 pounds of manganese; annual U. S. steel industry consumption approximates 1.5 million short tons manganese ore averaging 48% manganese.
Chemical, dry cell battery, other industries use another 80,000 to 100,000
tons of ore (857o manganese dioxide plus special purity requirements).
Today, with steel operating below capacity, some imported manganese ore is being stockpiled.
U. S. manganese deposits are uniformly low grade (5 to 20% manganese); are sufficient for a century or longer if developed. Marketable concentration is 40%, not within reach with classical physical ore dressing methods which can strain to 20 to 25%.
New method, developed by Ernest S. Nossen and pilot-planted by E. S. Nossen Laboratories, utilizes a nitric acid cycle, can produce saleable iron, calcium nitrate fertilizer as by-products.
Process separates manganese from silica, other undesirable impurities including iron. Nitric acid can be re
covered at better than 95%, an essential step which makes process economic.
Use of Maine Ore. Raw material for Paterson pilot plant is low grade ore from Aroostook County, Maine, running about 10 to 12% manganese. It contains large amounts of calcium oxide, iron oxide, silica, so intimately mixed that ore has always been considered useless.
Local ore variations may require small changes in treatment of each batch.
Ore, in 4000 pound batches, i s crushed and ground to —60 mesh. I n a multiple hearth furnace, between 600° and 900° C , it is reduced in a reducing atmosphere formed by incomplete combustion of city gas. Manganese dioxide goes to the monoxide, iron oxide goes to ferrosoferric oxide. Mixture is then leached with nitric acid diluted with wash water from previous batches. Nitric acid concentration in leach is 20 to 25%; when ore is present actual free acid concentrja-tion is lowered.
insoluble ferrosoferric oxide, silicon dioxide, aluminum oxide are filtered
4 4 2 0 C H E M I C A L A N D E N G I N E E R I N G N E V / S

Manganese nitrate is decomposed directly to manganese dioxide and nitric acid in this stainless steel drum decomposer in Έ. S. Nossen Laboratories' pilot plant at Paterson, N. J. This pilot plant is processing low grade domestic ores at the rate of 1000 pounds an hour. It produces a concentrate containing 60% manganese from ore containing 5 to 20%. Concentrate may compete with imported high grade ores
off from soluble manganese nitrate, alkaline earth salts. Reduction step is not necessary when manganese is present in ore as monoxide or carbonate, as in Maine ore.
If the ore contains relatively little calcium, sulfuric acid is used to precipitate calcium, barium, and lead. Where large quantities of calcium are present it is more economical to skip this step and recover calcium nitrate for fertilizer applications. Most ore deposits are convenient to fertilizer markets, an advantage which might warrant installation of nitric acid manufacturing facilities.
Residue is washed for maximum recovery of soluble manganese; solution is concentrated from about 40% to 70% solids in an evaporator. Decomposition directly to manganese dioxide and nitric acid is carried out in a drum decomposer in presence of steam and air at about 200° C. Dioxide is washed; if sulfuric precipitation of alkaline earth nitrates has been skipped, earth nitrates are washed out and recovered. In a full scale production plant vacuum filters would be replaced by countercurrent thickeners.
Nitric acid vapors and some oxides of nitrogen from the drum decomposer are recovered by a condenser and absorption system. Iron can be salvaged by magnetic separation.
Break-Even at 100 Tons. Breakeven point is about 100 tons of concentrate per day, or 600 to 700 tons of ore. Process is being encouraged by the Government and also by local interests in Maine, where stable indus
try is badly needed. Unnecessary handling costs can be avoided by locating close to a mine; markets for by-product fertilizer will then also be convenient.
Difficult operating conditions—gas reactions, high temperatures, pressures—are avoided; standard mining, metallurgical, chemical equipment can be used. Stainless steel leaching, decomposition equipment eliminate corrosion problems.
Pyrometallurgical, other chemical concentration processes are only economic in extreme national emergencies. Chemical processes are only feasible when chemicals are recovered (as in this case) or when chemical consumption is negligible (as in case of Montana carbonate ores, which can be calcined after physical concentration to give 60% concentrate). Nossen process is applicable to carbonate, oxide, several types of silicate ores. Product is suitable for both chemical and dry cell battery industries.
Vapors of nitric acid and N02 from the drum decomposer are condensed and collected in condenser and absorption towers. More than 95% of the acid is recovered making process economic τ
V O L U M E 3 2, N O . 4 4 · N O V E M B E R I, 1954 4421